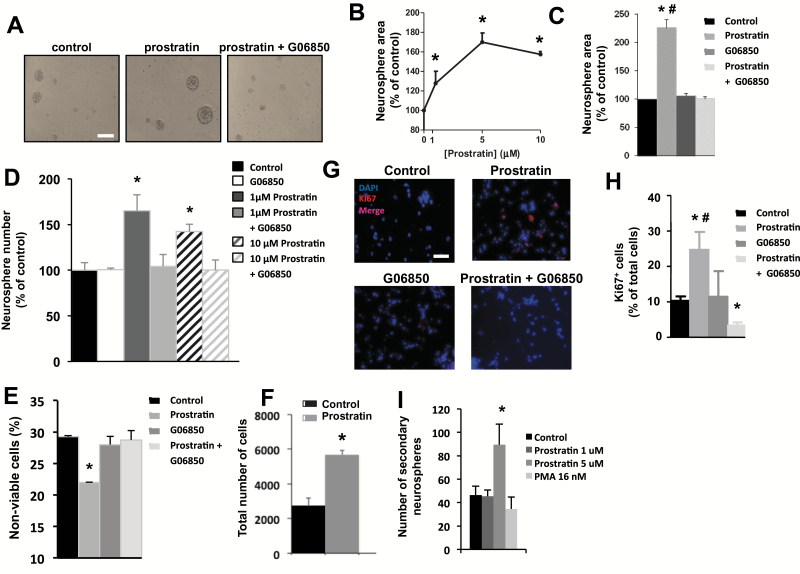
Figure 2.
Effect of prostratin on neural progenitor cell (NPC) proliferation in vitro. Proliferation was tested in NPC cultures grown in the presence of basic fibroblast growth factor (bFGF). (A) Phase-contrast microscopy images of neurospheres cultured for 72 hours in the absence or presence of prostratin (5 µM) with or without addition of the PKC inhibitor G06850 (0.5 µM). Scale bar indicates 200 µm. (B) Graph shows the effect of increasing concentrations of prostratin on neurosphere area after 72 hours of culture. (C) Graph shows neurosphere area after 72 hours of treatment with or without 5 µM prostratin and/or 0.5 µM G06850. (D) Effect of prostratin on neurosphere number, counted 72 hours after seeding, in the absence or presence of 0.5 µM G06850. (E) Percentage of nonviable cells quantified by trypan blue exclusion after 48 hours of culture in the presence or absence of 5 µM prostratin and/or 0.5 µM G06850. (F) Total cell number in NPC cultures after 72 hours in the presence or absence of 5 µM prostratin. (G) Fluorescence microscopy images of neurosphere-derived adhered cells grown for 48 hours with or without 5 µM prostratin and/or 0.5 µM G06850. Cells were immunostained to detect Ki67, and nuclei were counterstained with 4’,6-diamidino-2-phenylindole. Scale bar indicates 100 µm. (H) Quantification of Ki67+ cells in cultures described in G. (I) Effect of a 72-hour pretreatment of NPC with prostratin or PMA (at the indicated concentrations) on the number of secondary neurospheres formed in the following 72-hour subculture in the absence of drugs. *P<.05 compared with control in a Student’s t test; #P<.05 compared with the other groups in ANOVA for repeated measurements, followed by Student’s t test.