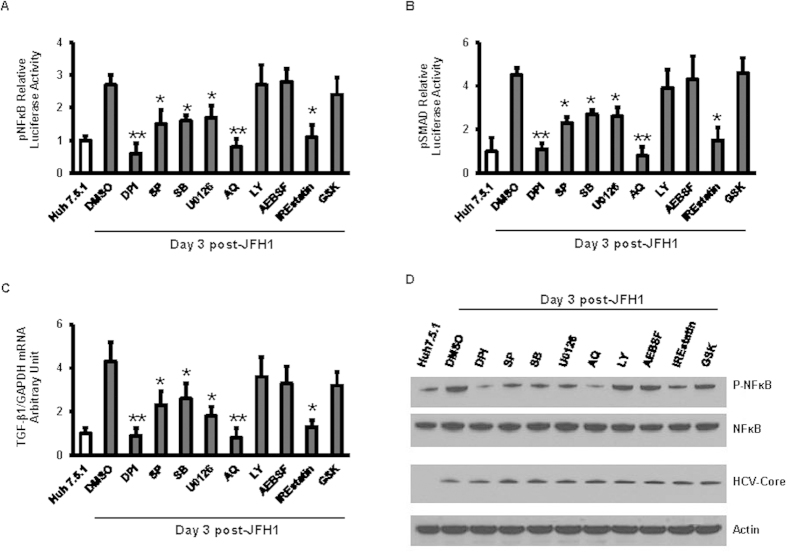
Figure 5. ROS, JNK, and IRE1 inhibitor decreased HCV activated NFκB and TGF-β1.
Huh7.5.1 cells and Huh7.5.1 infected with JFH1 for 3 days were transfected with plasmid pNFκB or pSMAD-luciferase reporter for 24 hours. The pRL-TK expression Renilla luciferase was co-transfected as an internal control. After the transfection, cells were treated with several pathway inhibitor including DPI, SB, SP, U0126, LY, AQ, AEBSF, IREstatin and GSK (20 μM). 1% DMSO was used as a negative control. Luciferase signaling was monitored by a dual luciferase reporter assay at 24 hour after inhibitor treatment. Relative luciferase activity (RLA) was normalized by dividing the firefly luciferase value by the Renilla luciferase value. DPI, SB, SP, U0126, AQ, or IREstatin treatment decreased HCV-induced NFκB and TGF-β1 promoter signaling compared with DMSO control in JFH1 cells (Fig. 5A,B). TGF-β1 mRNA level was determined by real-time PCR and normalized to GAPDH. We found that HCV-induced TGF-β1 mRNA expression enhancement was blunted by DPI, SP, SB, U0126, AQ or IREstatin treatment compared with DMSO control in JFH1 cells (Fig. 5C). Whole cell lysates were analyzed by western blot to detect NFκB phosphorylation. DPI, SP, SB, U0126, AQ and IREstatin decreased HCV induced NFκB phosphorylation in JFH1 cells (Fig. 5D). Lane 1, Huh7.5.1 + DMSO; lane 2, JFH1 + DMSO; lane 3, JFH1 + DPI; lane 4, JFH1 + SP; lane 5, JFH1 + SB; lane 6, JFH1 + U0126; lane 7 JFH1 + AQ; lane 8, JFH1 + LY; lane 9, JFH1 + AEBSF; lane 10, JFH1 + IREstatin; lane 11, JFH1 + GSK. *p < 0.05 and **p < 0.01 for comparison of indicated and JFH1-infected cells.