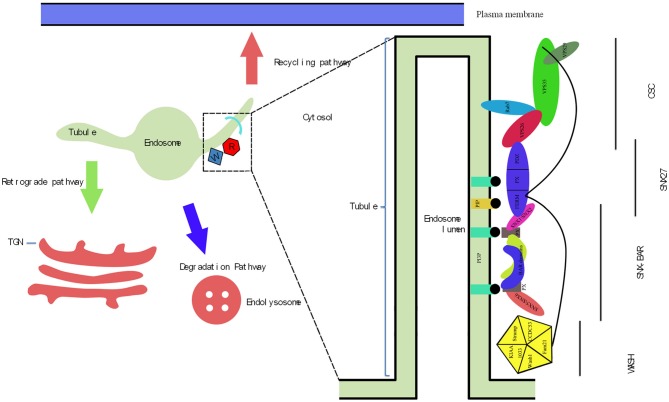
Figure 1.
The endosomal trafficking managed by retromer and the molecular architecture of inter-relationship between the retromer complex, sorting nexin family member 27 (SNX27) and Wiskott-Aldrich syndrome protein and scar homolog (WASH) complex. (1) There are three trafficking pathways regulated by retromer. Initially, the retrograde pathway, cargo proteins are retrieved from the endosomes and trafficked to the trans-Golgi network (TGN). The second is the recycling pathway in which cargo proteins are trafficked to the plasma membrane (PM) for reuse. The first two transport routes above both via tubules depended that extend out of edosomal membranes. The last pathway is degradative pathway, which helps in internalizing cargoes to the endosome then gradually matures to become the multi-vesicular late endosome (LE) before fusing to existing lysosomes resulting in the degradation of cargoes in the endolysosome. (2) The endosomal-sorting complex is composed of retromer complex, SNX27 and WASH complex. The WASH complex is comprised of five proteins: KIAA1033, Strumpellin, FAM21, WASH1, and Coiled-Coil Domain Containing 53 (CCDC53). SNX27 is composed of protein-interaction domains often found in multi-domain scaffolding proteins known as PDZ domain, the phoxhomology (PX) domain and the FERM domain. Retromer complex consist of cargo-selective complex (CSC; VPS35, VPS26 and VPS29) and SNX-BAR (SNX1 or SNX2 and SNX5 or SNX6). VPS35 supports a platform that VPS26 and VPS29 bind to it. Rab7 interacts with VPS26 and VPS35 as well as recruits the CSC to the endosome membrane. VPS26 binds to the PDZ domain of SNX27. The FERM-like domain of the SNX27 directly interacts with SNX1 or SNX2 of SNX-BAR. The recruitment of SNX27 and SNX-BAR to endosomes is both through the binding of phosphatidylinositol-3-phosphate (PI3P) by their PX domain. In addition, within the FERM domain of SNX27, is a second site with high affinity for PIP. As for the WASH complex, which is responsible to link itself to the VPS35, VPS29 and SNX27 FERM domain through its unstructured C-terminal tail in FAM21 and is recruited by CSC.