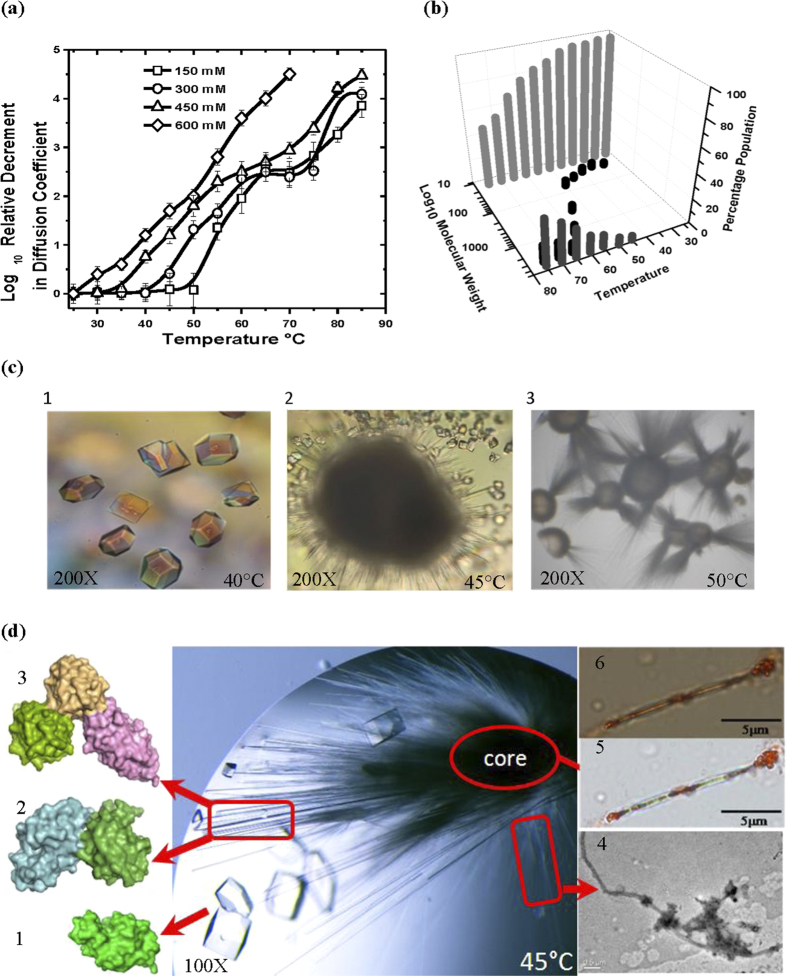
Figure 1. Tracking the increase in particle size of lysozyme as a function of heating.
(a) Relative change in diffusion coefficient at 20 mg/ml protein concentration on heating lysozyme from 20 to 85 °C in 150 mM (-◻-), 300 mM (-○-), 450 mM (-Δ-) and 600 mM (-§-) NaCl at pH 3.8 is presented here. (Lines are spline curves plotted to guide the eye on the trend). (b) SEC-MALLS data as a function of temperature showing monomeric ( ), dimeric and trimeric associated states (
), dimeric and trimeric associated states ( ), and higher order aggregates (
), and higher order aggregates ( ) in solution of lysozyme incubated at different temperatures. (c) Crystallization of lysozyme at elevated temperatures. Tetragonal crystals at 40 °C (1), drop contained needle like crystals emerging out spherulite and normal tetragonal crystals at 45 °C (2), spherulites formed in crystal drop at 50 °C (3). (d) Crystallization drop containing spherulite and usual tetragonal crystals of HEWL formed at 45 °C. Tetragonal crystals were composed of monomeric protein (1), needle shaped crystalline outgrowths were composed of natively associated protein molecules (2,3), thin spikes analyzed using TEM were composed of amyloid fibrils (4), samples taken from amorphous core get stained with Congo red dye (5) emit apple-green birefringence pattern when visualized under cross polarizers thus confirms that core is composed of amyloid fibrils (6).
) in solution of lysozyme incubated at different temperatures. (c) Crystallization of lysozyme at elevated temperatures. Tetragonal crystals at 40 °C (1), drop contained needle like crystals emerging out spherulite and normal tetragonal crystals at 45 °C (2), spherulites formed in crystal drop at 50 °C (3). (d) Crystallization drop containing spherulite and usual tetragonal crystals of HEWL formed at 45 °C. Tetragonal crystals were composed of monomeric protein (1), needle shaped crystalline outgrowths were composed of natively associated protein molecules (2,3), thin spikes analyzed using TEM were composed of amyloid fibrils (4), samples taken from amorphous core get stained with Congo red dye (5) emit apple-green birefringence pattern when visualized under cross polarizers thus confirms that core is composed of amyloid fibrils (6).