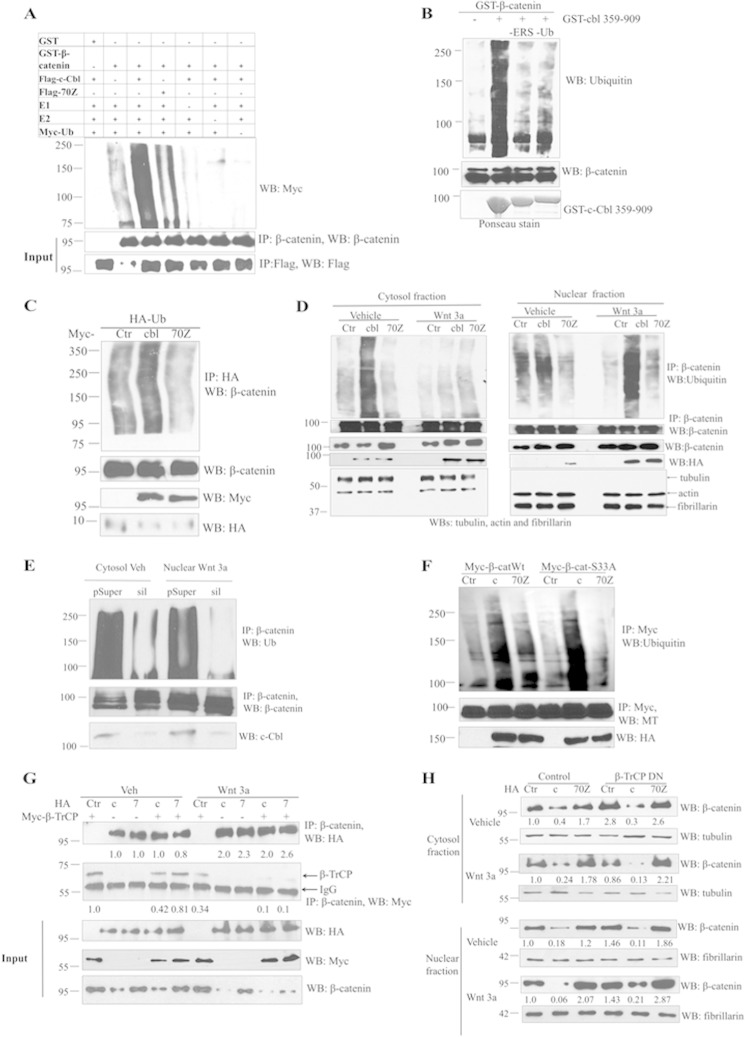
FIGURE 4.
c-Cbl ubiquitinates β-catenin in both the phases of Wnt signaling. A, in vitro ubiquitination of β-catenin by c-Cbl. Ubiquitination reaction mixture was reconstituted in vitro using GST or GST-β-catenin and IPed FLAG-c-Cbl or c-Cbl-70Z from ECs along with 225 nm E1-activating enzyme, 500 nm E2 conjugase, 600 μm Myc-ubiquitin, 1 mm MgCl2-ATP and incubated at 37 °C for 60 min. Reactions without E1, E2, c-Cbl or ubiquitin served as negative control. β-Catenin cleaved from GST beads using thrombin and then eluent were immunoblotted using Myc antibody. The blot was stripped and reprobed using β-catenin and FLAG antibodies. Representative immunoblot of two experiments is shown. B, ex vivo ubiquitination of purified β-catenin using the HeLa cell cytosolic S100 fraction. Purified recombinant GST-β-catenin on glutathione-SepharoseTM beads was incubated with HeLa cell S100 fraction (pretreated with ubiquitin aldehyde and MG132), Myc-tagged human recombinant ubiquitin (Ub), recombinant GST-tagged c-Cbl(359–909), and energy regeneration solution. Eluent was resolved with SDS-polyacrylamide gel. The nitrocellulose membrane was stained with Ponceau stain for c-Cbl input and immunoblotted using monoclonal ubiquitin and reprobed using β-catenin antibodies. Lanes designated as -ERS or -UB were reactions without energy-regenerating solution (ERS) or ubiquitin only, respectively. Representative immunoblot was from two experiments. C, c-Cbl, but not 70Z enhances β-catenin ubiquitination. HEK293T cells cotransfected with HA-Ub and Myc empty vector or c-Cbl or 70Z were treated with 10 μm of MG132 for 16 h. The cells were then lysed in RIPA buffer and immunoprecipitated with HA antibody and immunoblotted using β-catenin antibody. Five percent of cell lysates were probed as input. Representative image of two experiments is shown. D, c-Cbl ubiquitinates cytosolic β-catenin in Wnt-off phase and nuclear β-catenin in Wnt-on phase. Human aortic endothelial cells stably expressing control (Ctr), HA-tagged c-Cbl (cbl), or c-Cbl-70Z pretreated with MG132 at 10 μm for 12 h were subjected to subcellular fractionation and IP as above. The membrane was stripped and reprobed with β-catenin antibody. α-Tubulin and fibrillarin served as markers of cytosolic and nuclear fractions, respectively. Representative immunoblot from two experiments is shown. E, c-Cbl silencing reduces β-catenin ubiquitination in both the phases of Wnt signaling. 500 μg of cytosolic or nuclear lysates of ECs cells transduced with control (pSup) or c-Cbl silencing (sil) and serum-starved and treated with vehicle or Wnt3a were processed as above. Because both β-catenin and c-Cbl are predominantly located in the cytosol in Wnt-off and in the nucleus in Wnt-on phase (Fig. 2A), the respective fractions are shown. The blot was stripped and reprobed for β-catenin. Ten percent of cell lysates were probed for endogenous c-Cbl. Representative immunoblot from three experiments is shown. F, c-Cbl ubiquitinates naturally occurring oncogenic mutant β-catenin S33A. Lysates of HEK293T cells stably expressing HA-tagged c-Cbl and transiently transfected with Myc-tagged β-catenin and treated with MG132 at 10 μm for 12 h were subjected to IP using 0.5 μg of Myc antibody. The eluents were probed with ubiquitin antibody and reprobed with Myc antibody. Representative immunoblot of two experiments. G, both c-Cbl and β-TrCP bind β-catenin in Wnt-off, whereas only c-Cbl binds to β-catenin in Wnt-on phase. HEK293T cells stably expressing HA c-Cbl (c) or c-Cbl-70Z (7) and transiently coexpressing Myc-β-TrCP were serum-starved for 16 h, treated with vehicle or Wnt3a (50 ng/ml) for 3 h, and IPed using β-catenin antibody and immunoblotted with HA and Myc antibodies. The co-IPed c-Cbl and β-TrCP were normalized to immunoglobulin. Five percent of lysates are shown as inputs. Representative immunoblot of three experiments is shown. H, β-TrCP regulates β-catenin in Wnt-off, whereas c-Cbl regulates β-catenin in both the phases of Wnt signaling. HEK293T cells stably expressing HA c-Cbl (c) or c-Cbl-70Z (7) and transiently coexpressing Myc-dominant negative β-TrCP (DN) were serum-starved for 16 h and treated with vehicle or Wnt3a (50 ng/ml) for 3 h and fractionated and probed with endogenous β-catenin; tubulin and fibrillarin served as cytosolic and nuclear markers, respectively, and as loading controls. Representative immunoblot of three experiments is shown.