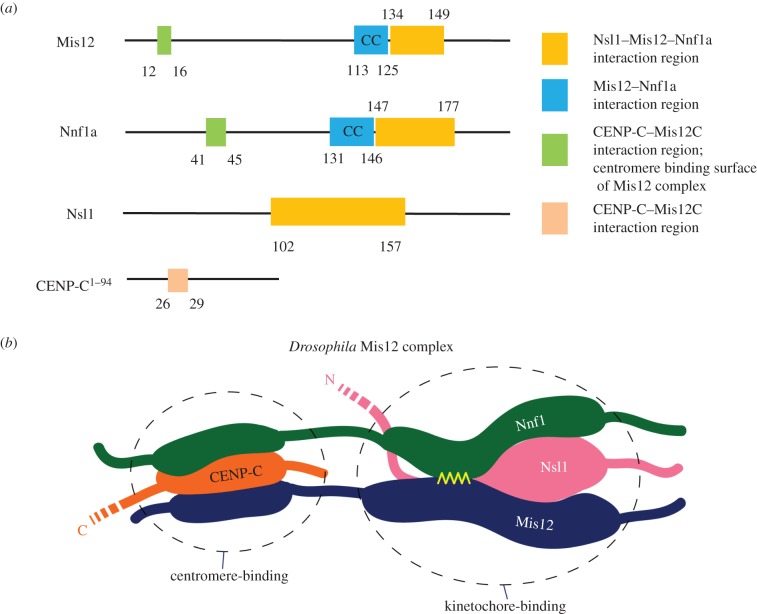
Figure 8.
Mis12–Nnf1a form a platform for interaction with CENP-C and Nsl1. (a) Schematic of interaction regions in CENP-C, Nnf1a, Mis12 and Nsl1. Coloured boxes show protein regions that were identified as interacting surfaces in this study. (b) Cartoon showing the hypothetical organization of the Drosophila Mis12 complex emerging from this study. Amino-terminal ends of the MN dimer (the centromere-binding part) are anchored at the centromeric chromatin via CENP-C. Carboxy-terminal fragments of the MN dimer (the kinetochore-binding part) serve as scaffold for binding outer kinetochore components, including Spc105/KNL1 and the Ndc80 complex. The yellow ribbon represents the CC interaction between Mis12 and Nnf1.