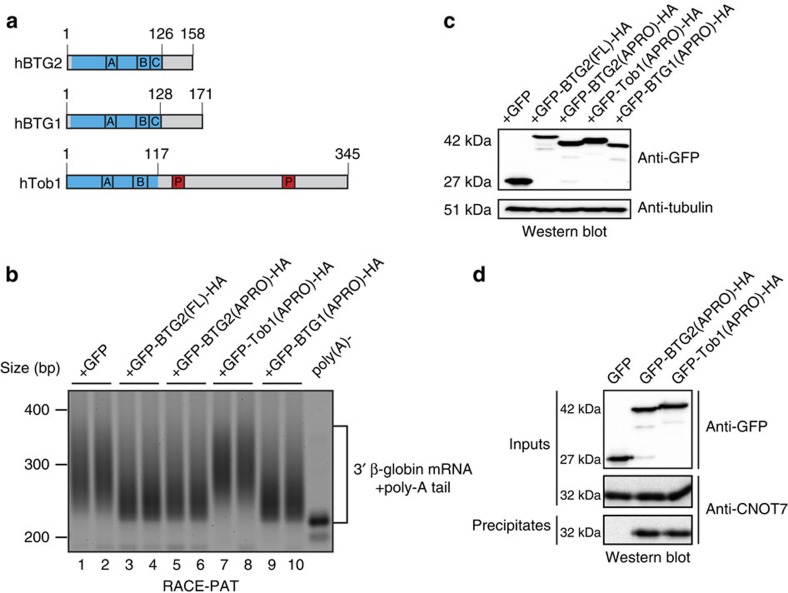
Figure 1. APRO domains of BTG2 and BTG1 but not of Tob1 are sufficient to stimulate deadenylation of a reporter transcript.
(a) Schematic organization of human BTG2, BTG1 and Tob1 proteins. APRO domains are highlighted in blue. The conserved boxA (A) and boxB (B) motifs (signatures for the BTG/Tob family), the boxC motif (C; specific for BTG1 and BTG2, see Supplementary Fig. 7) and the PAM2 motifs (P; found in the C-terminal region of Tob1) are indicated. Sequence identity/similarity between hBTG2 and hBTG1 APRO domains are 68.0%/79.7%, respectively, while sequence identity/similarity between hBTG2 and hTob1 APRO domains are 38.6%/58.3% respectively. (b) Reverse transcription polymerase chain reaction amplification of the poly(A) tails of the β-globin reporter transcript. HEK293 Tet-Off cells were co-transfected with plasmids expressing the β-globin reporter and the different HA-tagged BTG/Tob GFP fusion proteins (or empty GFP expression vector). Fragments comprising ∼200 bp of the 3′ end of the β-globin mRNA in addition to poly(A) tails were amplified using a RACE-PAT assay. An RNA sample treated with oligo(dT) and RNase H was used as a control for deadenylated β-globin mRNA (poly(A)-). Experiment was repeated twice with two biological duplicates. Quantification of the gel gave an estimated average poly(A) tail length (mean±s.d., n=2) of 74.8±1.6, 43.3±1.1, 46.8±3.7, 79±3.4 and 37.4±0.2 nucleotides in cells expressing GFP, GFP-BTG2(FL)-HA, GFP-BTG2(APRO)-HA, GFP-Tob1(APRO)-HA and GFP-BTG1(APRO)-HA, respectively. (c) A western blot analysis of the cell lysates that corresponded to the transfections performed in b is shown to demonstrate expression of the GFP fusion proteins. (d) Co-immunoprecipitation of CAF1 (CNOT7 paralogue) with BTG2 and Tob1 APRO domains. HEK293 cells were transfected with plasmids expressing HA-tagged BTG/Tob GFP fusion proteins or empty GFP expression vector. Proteins were precipitated with anti-HA agarose beads and revealed by western blot with anti-GFP and anti-CNOT7 antibodies.