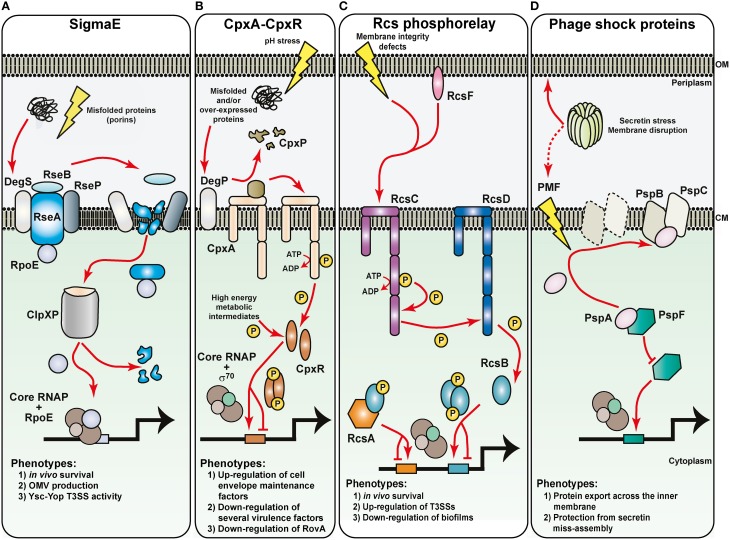
Figure 4.
Sensing of noxious extracytoplasmic stress by the bacteria envelope of Yersinia. The molecular basis for the activation of four prominent extracytoplasmic stress sensing sentinels are displayed, along with a summary of their respective phenotypic effects in pathogenic Yersinia. Maintaining the outer membrane (OM) are the RopE-, Cpx-, and Rcs-pathways. (A) Outer membrane protein misfolding initiates digestion of the anti-RpoE factor, RseA, through successive proteolytic actions of the DegS, RseP, and ClpXP proteases. Free RpoE is released into the cytoplasm, and when engaged with core RNA polymerase (RNAP), can establish controlled transcription of a large RpoE-regulon. The Cpx two-component system (B) and the Rcs phosphorelay system (C) both rely upon sensor kinase autophosphorylation (CpxA and RscC, respectively) to initiate the transduction of phosphate through to the cytoplasmic cognate response regulator (CpxR and RcsB, respectively). CpxA activation requires the DegP-dependent release of inhibitory CpxP, while RcsC activation might utilize an RcsF-dependent pathway. Inorganic phosphate can also be donated from unstable high-energy metabolic intermediates. Active phosphorylated response regulators dimerize and in concert with the house-keeping RNAP holoenzyme, target specific responsive promoters to influence transcriptional output. RcsB transcriptional control sometimes requires partners with RcsA. (D) Controlling the integrity of the cytoplasmic membrane (CM) are the phage shock proteins (psp). Secretin complex mislocalization to the cytoplasmic membrane risks dissipating the proton motive force (PMF). This is prevented by the PspB and PspC proteins actively sequestering the anti-PspF factor, PspA, to free up the PspF transcription factor to initiate promoter targeting and transcriptional output by the house-keeping RNAP holoenzyme.