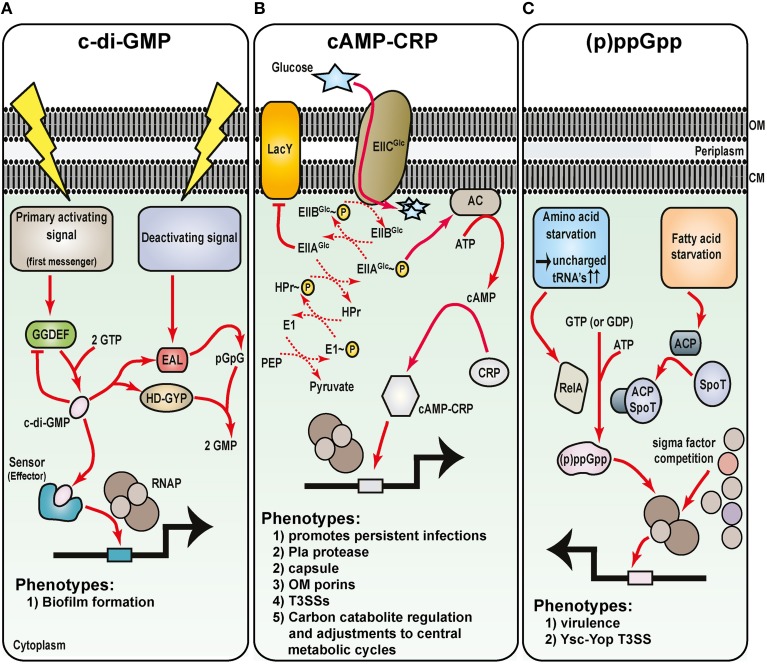
Figure 5.
Metabolic intermediates regulate Yersinia virulence. Understanding the role of nucleotide-based second messengers in the regulation of Yersinia survival and virulence is still in its infancy. As determined from many studies in E. coli systems, three major regulatory molecules are known: (A) c-di-GMP, (B) cAMP and its receptor Crp, and (C) ppGpp and pppGpp [collectively known as (p)ppGpp]. Primary activation signals control diguanylate cyclase (GGDEF domain containing proteins) to generate c-di-GMP (A). Various effector molecules then interact with c-di-GMP to regulate gene expression, including Hms-mediated biofilm formation. Deactivating signals stimulate phosphodiesterase activity of proteins containing either EAL or HD-GYP domains, and this degradation pathway ensures that c-di-GMP levels are stringently controlled. Glucose availability and the phosphoenolpyruvate (PEP)—carbohydrate phosphotransferase system (PTS) control cAMP production by adenylate cyclase (AC) (B). Upon cAMP production when glucose is limiting, cAMP-CRP complexes form and this enhances RNAP holoenzyme binding to vast numbers of sensitive promoters including several secreted Yersinia virulence factors. Finally, various forms of starvation stimulates RelA-dependent and SpoT/acyl carrier protein (ACP)-dependent synthesis of (p)ppGpp (C). By direct binding to the RNA polymerase, (p)ppGpp can influence sigma factor competition for core RNAP, and through this affects transcription of a plethora of genes that in Yersinia includes the prominent Ysc-Yop plasmid-encoded T3SS.