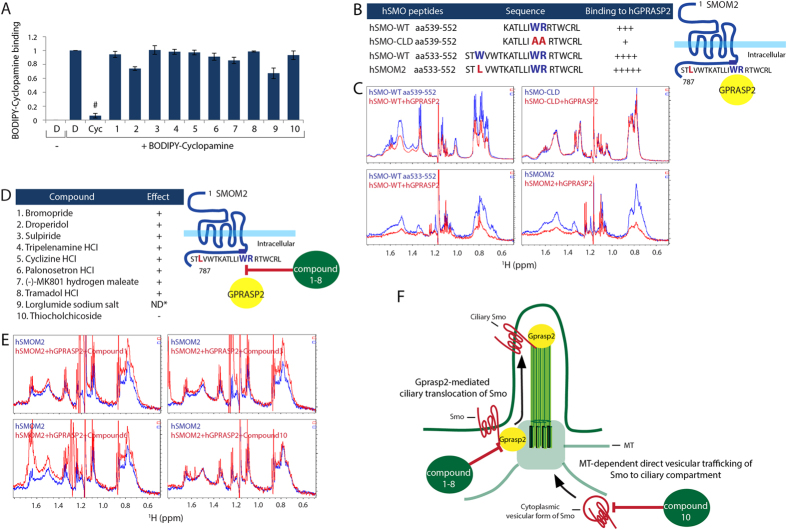
Figure 3. Identification of novel mechanisms of known small molecules in Hh signaling pathway.
(A) Compound competition assay with fluorescence BODIPY-Cyc for Smo binding in HEK293T cells as monitored by FACS analysis. All error bars represent the mean SD of three independent experiments. A two tailed unpaired t-test is used for statistical data analysis. (#p < 0.001) D = DMSO, 0.1%; BODIPY-Cyc, 20nM; Cyc, 10 μM; compound 1–10, 10 μM (B) The left panel of the table presents NMR data summaries of binding specificity of hGPRASP2 to different hSMO peptides harboring the helix VIII. The right panel shows a schematic illustration of SmoM2 and Gprasp2 interaction. (C) Selected aliphatic region on 1H NMR spectra of the different hSMO peptides were acquired before (blue line) and after addition of full-length hGPRASP2 (red line). (D) The left panel of the table shows NMR data summaries of compound interference of hGPRASP2 binding to hSMOM2. The right panel presents a schematic illustration of compound 1–8 effects on hSMOM2 and hGPRASP2 interaction. (E) Selected aliphatic region on 1H NMR spectra of the hSMOM2 peptide were acquired before (blue line) and after addition of full-length hGPRASP2 with compounds (red line). Note that compound 9 caused hSMOM2 peptide and hGPRASP2 protein precipitation at the experimental concentration. Therefore, it was not able to determine the compound action on the SmoM2 binding to Gprasp2. (ND*, not determined) (F) Hypothetical model of compounds’ action on Smo trafficking to the PC. Compounds 1–8 directly inhibit Smo and Gprasp2 interaction that is important for ciliary Smo localization. Compound 10 inhibit direct vesicular trafficking of Smo to ciliary compartment by disrupting MT network (Supplemental Fig. 5E).