Fig. 9.
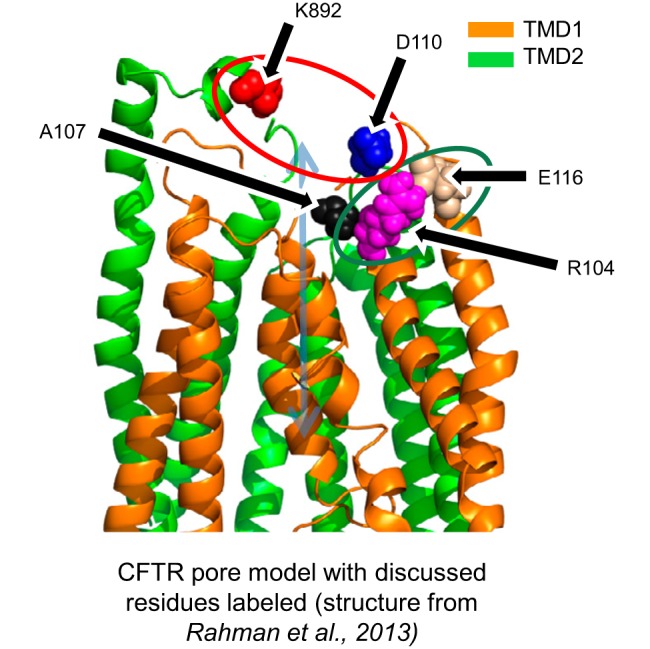
Structural interpretation of cross-linking results toward a model of CFTR gating. Structural model is built from coordinates of a 2.5-ns snapshot of a molecular dynamics simulation of CFTR previously published by our group (32). This study suggests that close proximity between cysteine-substituted residues at positions 892 and 110 (red circle) stabilizes a closed state of the pore, while we previously demonstrated that close proximity between cysteine residues at positions 104 and 116 (green circle) stabilizes an open state of the pore. Both linkages modify pore-gating behavior by impacting the position of the extracellular end of the first transmembrane helix of CFTR, possibly by altering the position of A107 (black), an amino acid predicted to lie in the permeation pathway for chloride ions.
