Figure 1.
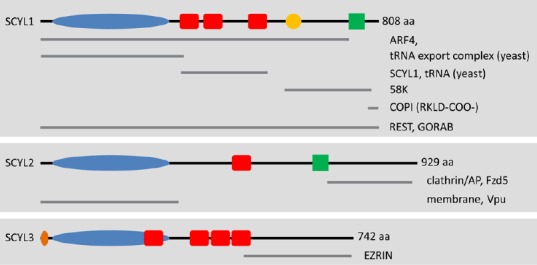
Schematic representation of SCYL family members and their potential interacting partners.
The SCYL family of protein pseudokinases comprises SCYL1, SCYL2, and SCYL3. Several SCYL1 isoforms have been reported in humans, most of which display variable C-terminal regions. These isoforms are not presented here. The kinase domains (blue ovals) of SCYL proteins are predicted to be inactive because of alterations of residues that are essential for catalytic activity. The red boxes represent the predicted HEAT repeats. The crystal structure of Cex1p (the yeast ortholog of SCYL1) reveals the presence of 6 pairs of antiparallel α-helices. The orange circle represents a proline-rich region of unknown function in SCYL1. The green boxes represent predicted coiled-coil regions. The orange oval represents the myristoylation consensus sequence found in SCYL3. Gray lines underneath the SCYL proteins highlight the regions of SCYL proteins that mediate interactions with their protein partners (labeled on the right of each gray line). For example, SCYL1 interacts with COPI via dibasic residues found in the C-terminal region of SCYL1 (RKLD-COO-). Other regions of SCYL1 are proposed to mediate the interactions with various other macromolecules, such as the small GTPases Arf4 and Ran (the tRNA export complex); tRNAs, Golgi-associated proteins GORAB and 58K; and itself, SCYL1. Homo-oligomerization of SCYL1 and tRNA binding are mediated by the central HEAT repeats. The N-terminal pseudokinase domain of SCYL2 can interact with the HIV protein Vpu and is capable of interacting with membrane structures within cultured cells. The C-terminal region of SCYL2 can also interact with clathrin/AP complexes (AP1 and AP2) as well as Fzd5. The C terminus of SCYL3 mediates the interaction between SCYL3 and the membrane-associated protein EZRIN. The functional relevance of these interactions and their implications in SCYL proteins functions in vivo (in particular SCYL1 and SCYL2) is unclear. NextProt (http://www.nextprot.org/db/) was used to identify domains and regions of the human SCYL proteins.
aa: Amino acids; ARF4: ADP-ribosylation factor 4; COPI: coat protein complex coatomer I; REST: RE-1 silencing transcription factor.
