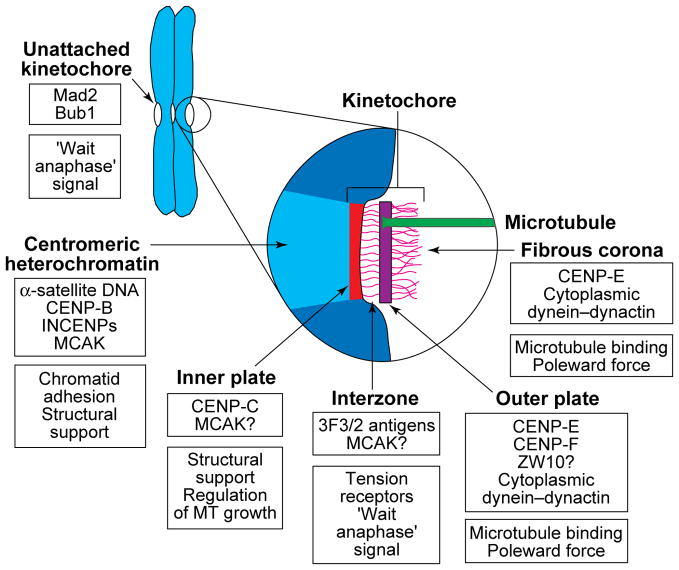
FIGURE 1.
A diagram depicting the putative location and possible function of reported kinetochore proteins. Sister kinetochores are located on opposite sides of the centromere region of replicated chromosomes, and the centromeric heterochromatin between them is rich in α-satellite DNA, its binding protein CENP-B62, and an inner centromere protein (INCENP)63 that might be involved in maintaining sister-chromatid cohesion. This region also appears to contain mitotic centromere-associated kinesin (MCAK)17, which has been shown in Xenopus extracts to be required for spindle formation and maintenance64. In electron micrographs of sections cut from conventionally fixed and stained preparations, the kinetochore region itself appears to consist of four structurally differentiated domains. The inner plate is closely associated with the centromeric heterochromatin and contains CENP-C, the presence of which is required for the maintenance of a functional kinetochore65, and perhaps MCAK. The zone between the inner and outer plates (the interzone) appears to contain the 3F3/2 phosphoepitope, which has been proposed to control the metaphase–anaphase (M–A) transition by sensing tension47, and MCAK might also be located in this region. Kinetochore microtubule (MT) plus-ends attach to and terminate at various levels within the outer plate, which has been reported to contain CENP-F22 (also called mitosin), CENP-E18–20, ZW1023 and possibly cytoplasmic dynein and its associated dynactin complex16. The fibrous corona extends from the outer plate and is apparent only on unattached kinetochores. It contains CENP-E20, ZW1023 and cytoplasmic dynein (reviewed in Ref. 16), the latter possibly being involved in MT attachment and poleward force production. Unattached (but not fully attached) kinetochores also contain Mad224 and Bub125 proteins, which are known to play important roles in regulating the M–A transition. A question mark indicates that the location of a protein is inferred from immunofluorescence microscopy rather than determined by immunoelectron microscopy.