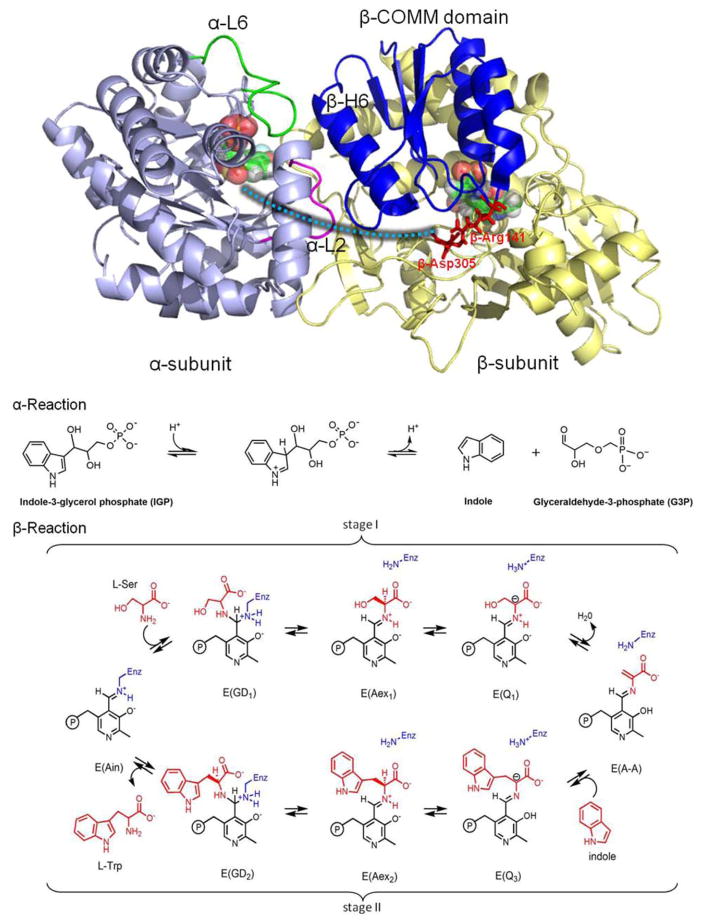
Figure 1.
Overall structure and chemical reactions of tryptophan synthase (TRPS). (top) TRPS is composed of an α-subunit (purple) and β-subunit (yellow). The two ligands binding to each subunit are shown in bead representation. The open, partially closed, and fully closed conformations of the α-subunit are controlled by αL2 (pink), αL6 (green), and βHelix-6 of the COMM domain (blue). The residues βArg141 and βAsp305, highlighted in red, are associated with the open and closed β-site conformations. The tunnel used to channel indole from the α-site to the β-site is marked as a cyan dashed line. (bottom) The α- and β-reactions of TRPS.