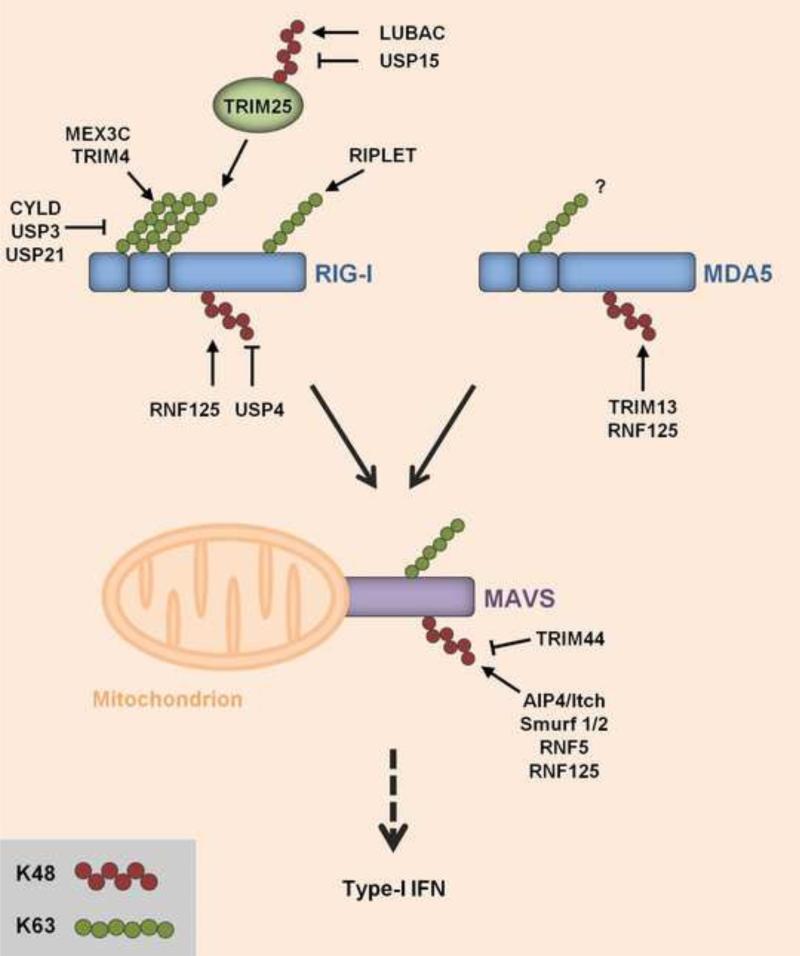
Figure 2. Regulation of RLRs by ubiquitination.
RIG-I and MDA5, members of the RIG-I-like receptor (RLR) family, recognize cytoplasmic viral RNA species and subsequently signal through the adaptor protein MAVS (also called Cardif, IPS-1, or VISA) on mitochondria. Through various steps (not illustrated), MAVS activates downstream signaling, leading to gene expression of type-I IFNs (IFN-α/β). The stability and signaling activities of RIG-I, MDA5 and MAVS are tightly regulated by K48- and K63-linked polyubiquitination, respectively. RIG-I is activated by K63-linked ubiquitination mediated by TRIM25, an IFN-inducible E3 ubiquitin ligase belonging to the large family of TRIM proteins. TRIM25 ubiquitinates several lysines in the N-terminal caspase activation and recruitment domains (CARDs) of RIG-I (not illustrated). TRIM25-mediated ubiquitination specifically at K172 in RIG-I is critical for RIG-I signaling. In addition, TRIM4 and MEX3C were shown to induce K63-linked ubiquitination of the RIG-I CARDs. Furthermore, RIPLET induces K63-linked ubiquitination of K788 (and also other residues) in the C-terminal domain (not illustrated). Both RIG-I and MDA5 have been reported to non-covalently bind unanchored K63-linked ubiquitin chains in vitro; however, the role of MDA5 activation by K63-linked ubiquitin chains remains unclear. TRIM25 itself undergoes K48-linked ubiquitination catalyzed by LUBAC, leading to TRIM25 degradation. Inversely, USP15 antagonizes the LUBAC-induced K48-linked ubiquitination of TRIM25, thereby stabilizing TRIM25 during viral infection, which leads to a sustained IFN response. MAVS is ubiquitinated with K63-linked polyubiquitin chains; however, the E3 ligase for MAVS K63-ubiquitination is unknown. Multiple different E3 ubiquitin ligases have been reported to induce the K48-linked ubiquitination of RLRs and MAVS, triggering their degradation by the proteasome: RNF125 for RIG-I; TRIM13 and RNF125 for MDA5; and AIP4/Itch, Smurf1/2, RNF5 and RNF125 for MAVS. The K48-linked ubiquitination of RIG-I can be actively removed by USP4. Furthermore, TRIM44, an atypical TRIM protein that lacks the RING E3 ligase domain, inhibits the K48-linked ubiquitination of MAVS through an unidentified mechanism.