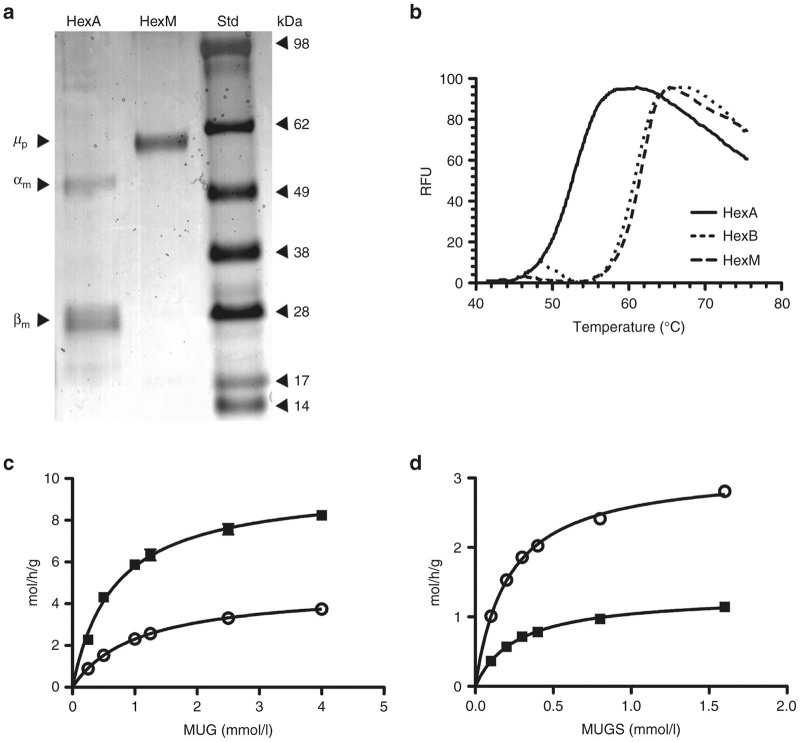
Figure 4.
Characterization of the heat stability and kinetic properties of HexM as compared to other Hex isozymes. (a) Protein stained SDS-PAGE gel demonstrating the purity of secreted HexM after Ni-NTA chromatography. The secreted, purified HexM precursor polypeptide (µp) versus the major mature α- and β- polypeptides (αm, βm) of isolated placental HexA. Molecular weight standards (Std) are shown at the right. (b) Confirmation was obtained using differential scanning fluorimetry that the β-subunit interface was successfully transferred into the HexM protein structure. The Tm of HexA calculated from its heat denaturation curve (solid line) was significantly lower (~10 °C) than those calculated for HexB (dotted line) and HexM (dashed line), which were virtually identical. (c,d) Comparison of the kinetics of purified HexM versus HexA for artificial substrates. The Km and Vmax values of HexM (open circles) and HexA (filled squares) for the MUG (c) and MUGS (d) substrates were determined from the specific activities of each isozyme (y-axis) at various concentrations of each substrate (x-axis) and fitted to the Michaelis-Menten equation using Prism Graphpad.