Abstract
Objective
The incidence, clinical characteristics and outcomes of critically-ill, non-intubated patients with evidence of the acute respiratory distress syndrome (ARDS) remain inadequately characterized.
Design
Secondary analysis of a prospective observational cohort study.
Setting
Vanderbilt University Medical Center.
Patients
Among adult patients enrolled in a large, multi-intensive care unit prospective cohort study between the years of 2006 and 2011, we studied intubated and non-intubated patients with ARDS as defined by acute hypoxemia (PaO2/FiO2 ≤ 300 or SpO2/FiO2 ≤ 315) and bilateral radiographic opacities not explained by cardiac failure. We excluded patients not committed to full respiratory support.
Interventions
None.
Measurements and Main Results
Of 457 patients with ARDS, 106 (23%) were not intubated at the time of meeting all other ARDS criteria. Non-intubated patients had lower morbidity and severity of illness compared to intubated patients; however, mortality at 60 days was the same (36%) in both groups (P=0.91). Of the 106 non-intubated patients, 36 (34%) required intubation within the subsequent 3 days of follow-up; this “late” intubation subgroup had significantly higher 60-day mortality (56%) compared to both the “early” intubation group (36%, P<0.03) and to patients never requiring intubation (26%, P=0.002). Increased mortality in the “late” intubation group persisted at 2 years follow-up. Adjustment for baseline clinical and demographic differences did not change the results.
Conclusions
A substantial proportion of critically ill adults with ARDS were not intubated in their initial days of intensive care, and many were never intubated. Late intubation was associated with increased mortality. Criteria defining ARDS prior to need for positive pressure ventilation are needed so that these patients can be enrolled in clinical trials and to facilitate early recognition and treatment of ARDS.
Keywords: Acute lung injury, Acute respiratory distress syndrome, Early Acute Lung Injury, Intensive Care, Acute Respiratory Failure, Mechanical Ventilation, Clinical Outcomes, Critical Illness, Critical Care
INTRODUCTION
The acute respiratory distress syndrome (ARDS) was first described almost 50 years ago by Ashbaugh and colleagues in critically ill adults requiring mechanical ventilation.(1) Lacking a formal definition, several subsequent studies similarly described ARDS almost universally in mechanically ventilated patients in the intensive care unit. (2–5) The consensus definitions of ARDS that followed, including the American European consensus conference (AECC) definition of acute lung injury and ARDS in 1992 and the Berlin definition for ARDS in 2012, were created with a primary goal of standardizing the diagnosis of ARDS for multicenter treatment trials and epidemiologic studies, rather than to capture the entire spectrum of illness.(6, 7) As a result, modern epidemiologic studies and treatment trials of ARDS have continued to focus almost exclusively on intubated, mechanically ventilated patients with ARDS.(8–17) In fact, the most recent Berlin definition requires positive pressure ventilation for the diagnosis of ARDS.(7)
While this approach has facilitated improvements in ARDS management and reduced mortality, primarily through lung protective ventilation,(8, 15) treatment of ARDS remain largely supportive, and disease-specific efforts have failed in multicenter clinical trials.(18) The success of early goal-directed care in sepsis offers the possibility that targeted treatments in ARDS may offer greater benefit prior to the onset of mechanical ventilation-dependent respiratory failure, and the recent shift by the National Institutes of Health’s ARDS Clinical Trials Network to focus on prevention and early treatment reflects this approach.(19) Comprehensive characterization of ARDS in earlier and less severe stages may provide important avenues for improved diagnostic considerations and novel therapies. Nearly one-third of children with ARDS are not mechanically ventilated on initial diagnosis(20), and respiratory failure requiring invasive mechanical ventilation likely represents only the most severe subset of a larger clinical syndrome in children.(21–25) However, data are limited on the epidemiology and clinical outcomes of non-mechanically ventilated adults with ARDS.
The purpose of the present study was (1) to determine how frequently critically-ill patients otherwise meeting the clinical, chest radiographic and oxygenation criteria are not intubated at the time of meeting all other ARDS criteria, and (2) to evaluate the clinical outcomes among these patients, compared to patients who were intubated and mechanically ventilated on the first day of ARDS diagnosis.
MATERIALS AND METHODS
Subjects
We conducted a secondary data analysis on patients enrolled between January 2006 and February 2011 in a prospective cohort study entitled the Validation of biomarkers for Acute Lung Injury Diagnosis (VALID) study, a multi-intensive care unit (ICU) study at Vanderbilt University Medical Center (VUMC). Details of the VALID study have been described previously.(26–28) Briefly, adult patients admitted to the medical, surgical, trauma or cardiovascular intensive care units at VUMC were enrolled on the morning of ICU day 2. Study Day 1 was defined as the time between ICU admission and enrollment in the VALID study (~8 am on ICU day 2). Days 2, 3 and 4 are subsequent 24 hour periods. Exclusions included ICU stay greater than 48 hours prior to Vanderbilt ICU admission, uncomplicated overdose, severe chronic lung disease, plans to transfer out of ICU on ICU day 2 and non-mechanically ventilated or post-surgical patients in the cardiovascular ICU. Patients were otherwise enrolled independent of their mechanical ventilation requirements.
For the current study, we included patients with ARDS,(7) defined as the development of acute, bilateral pulmonary infiltrates (as determined by consensus of two trained physician reviewers) and hypoxemia (PaO2/FIO2 ≤ 300 mm Hg) not primarily due to heart failure or volume overload. Patients were included independent of requirement of positive-pressure ventilation requirement. Therefore, patients on supplemental oxygen via nasal cannula and facemask were included if they otherwise met the diagnosis of ARDS. We specifically included these patients so that we could focus this study on the clinical outcomes of patients with the clinical phenotype of ARDS who were, at least initially, not requiring intubation. For diagnosis, the ratio of pulse oximetric saturation to fraction of inspired oxygen (SpO2/FiO2) ≤ 315 was used as a validated surrogate for PaO2/FiO2 for diagnosis of ARDS among patients without an arterial blood gas measurement at the time of ARDS diagnosis.(29) The diagnosis of ARDS could be established at any time during the first four days in the ICU. Among non-mechanically ventilated patients with supplemental oxygen via nasal cannula, every additional liter of flow of oxygen per minute was estimated as an additional 0.04 FiO2 over atmospheric FiO2 of 0.21.(30, 31) For non-mechanically ventilated patients using facemask delivery of oxygen, the recorded supplemental FiO2 was recorded as the inspired FiO2. All ARDS determinations and determinations of mechanical ventilation status were made independently for each study day.
Patients were excluded for a “do not intubate” (DNI) order at the time of enrollment or if the primary ARDS risk factor was trauma, because the pathogenesis and prognosis of ARDS and the prevalence of intubation differed in trauma-related ARDS.(32) Among 2325 patients enrolled in VALID during the study period, there were 475 non-trauma patients with ARDS. An additional 18 patients were excluded due to an initial DNI order for a total of 457 patients included in this sub-cohort study (Figure 1).
Figure 1. Flow diagram of inclusion and exclusion criteria.
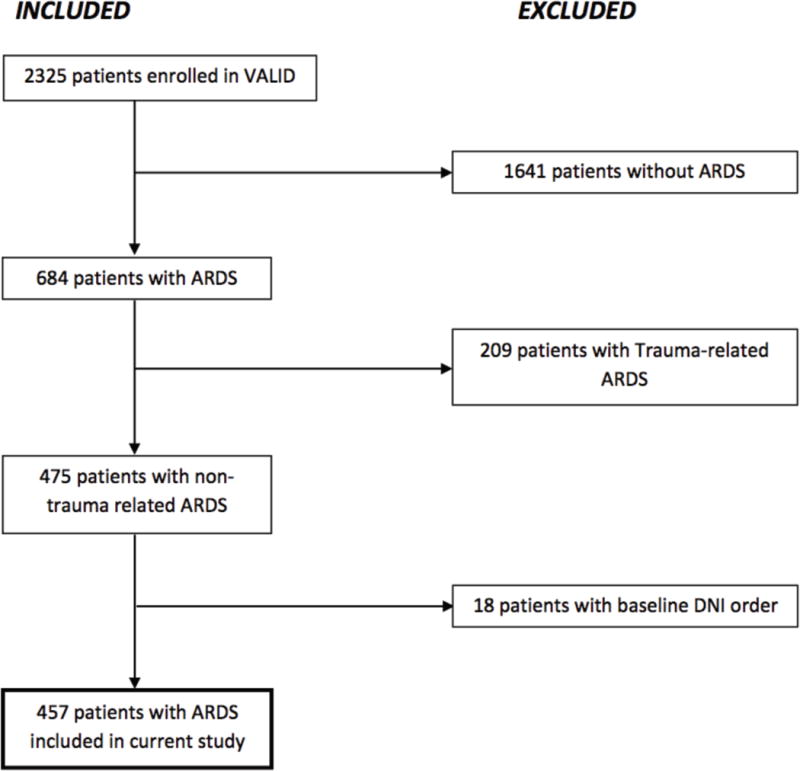
This flow diagram illustrates the total number of patients enrolled in the VALID study and the number and reasons for excluding patients based on our pre-determined criteria. Following this process, 457 patients with ARDS were identified for our study. Abbreviations: VALID=Validation of biomarkers in Acute Lung Injury Diagnosis; ARDS=Acute Respiratory distress syndrome; DNI=Do Not Intubate
The Institutional Review Board (IRB) at Vanderbilt University approved the study. Informed consent was obtained from patients or their surrogates whenever possible. For patients who were unable to provide informed consent due to their clinical condition and for whom no surrogates were available, a waiver of informed consent was granted by the IRB due to the minimal risk of the observational study.
Primary measures
The predictor variable was requirement of endotracheal intubation with positive pressure ventilation. We classified patients in the following two groups: (1) Early-intubation: Intubated/mechanically ventilated and meeting ARDS criteria on the same study day and (2) Initially non-intubated: Not requiring intubation on the day of meeting ARDS criteria. Patients receiving non-invasive positive pressure ventilation (NIPPV) at the time of meeting ARDS criteria were classified as non-intubated in the primary analysis. The initially non-intubated group was further subdivided into two subgroups: Never-Intubated: not requiring intubation on admission to ICU or at any time between study days 1 through 4 of follow-up; and Late-Intubation: not intubated on the day of ARDS diagnosis, but intubated on a subsequent study day.
The primary outcome variable was mortality at 60 days. Secondary outcomes were mortality at one and two years, 28-day ventilator-free days (VFD), defined as the number of days alive and free of mechanical ventilation to day 28, with VFD = 0 for patients who died in the first 28 days,(33) and the total number of ICU days in survivors to hospital discharge.
Covariates affecting intubation timing and status
We considered several baseline characteristics, comorbidities, clinical variables, severity of illness measures and initial process of care measures as possible factors influencing likelihood of and timing of intubation in acute lung injury as outlined in Tables 1 and 2. Organ failure was classified according to Brussels criteria: Coagulation failure defined as platelet count ≤ 80 × 103/mm3; renal failure defined as creatinine ≥ 2 mg/dL; circulatory failure defined as systolic blood pressure ≤ 90 mmHg and unresponsive to fluid; and hepatic failure-bilirubin ≥ 2 mg/dL.(34) The Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated using data from the 24 hours prior to enrollment.(35) Presence of consensus-defined sepsis was assessed daily for the first four study days.(36) Process of care measures included time from admission to ICU in days, fluid balance on day of ARDS diagnosis, and use of NIPPV at any point on the day meeting ARDS criteria.
Table 1.
Baseline Demographics and Co-morbidities According to Mechanical Ventilation Status among 457 Patients with ARDS
| All ARDS N=457
|
P-value | Initially Non-Intubated N=106
|
P-value | |||
|---|---|---|---|---|---|---|
| Early-Intubation N=351 | Initially Non-Intubated N=106 | Never-Intubated N=70 | Late-Intubated N=36 | |||
| Baseline Demographics | ||||||
| Age, Mean ± SD | 55 ± 16 | 54 ± 15 | 0.76 | 54 ± 16 | 56 ± 13 | 0.56 |
| Male | 174 (50) | 63 (59) | 0.08 | 46 (66) | 17 (47) | 0.07 |
| Race | 0.73 | 0.74 | ||||
| White | 298 (85) | 92 (87) | 60 (86) | 32 (89) | ||
| Black | 45 (13) | 13 (12) | 9 (13) | 4 (11) | ||
| Other | 8 (2) | 1 (1) | 1 (1) | 0 (0) | ||
| Source of Admission | <0.001 | 0.66 | ||||
| Emergency Department | 80 (23) | 38 (36) | 26 (37) | 12 (33) | ||
| Transfer from floor | 122 (35) | 48 (45) | 30 (43) | 18 (50) | ||
| Outside hospital | 94 (27) | 12 (11) | 7 (10) | 5 (14) | ||
| Operating room | 52 (15) | 7 (7) | 6 (9) | 1 (3) | ||
| Other | 3 (1) | 1 (1) | 1 (1) | 0 (0) | ||
| Current Smoker | 115 (33) | 31 (29) | 0.50 | 17 (24) | 14 (39) | 0.12 |
| Alcohol abuse | 57 (16) | 6 (6) | 0.006 | 3 (4) | 3 (8) | 0.39 |
| Illicit drug use | 27 (8) | 6 (6) | 0.48 | 4 (6) | 2 (6) | 0.97 |
| Co-morbidities | ||||||
| COPD | 54 (15) | 9 (9) | 0.07 | 6 (9) | 3 (8) | 0.97 |
| HIV | 12 (3) | 8 (8) | 0.07 | 6 (9) | 2 (6) | 0.58 |
| Diabetes | 107 (30) | 24 (23) | 0.12 | 16 (23) | 8 (22) | 0.94 |
| Cirrhosis | 32 (9) | 5 (5) | 0.15 | 1 (1) | 4 (11) | 0.03 |
| Congestive Heart Failure | 44 (13) | 10 (9) | 0.39 | 6 (9) | 4 (11) | 0.67 |
| Chronic kidney disease | 53 (15) | 18 (17) | 0.64 | 11 (16) | 7 (19) | 0.63 |
| Solid tumor (metastatic and non-metastatic) | 72 (21) | 16 (15) | 0.22 | 13 (19) | 3 (8) | 0.16 |
| Leukemia (chronic or acute) or stem cell transplant | 26 (7) | 25 (24) | <0.001 | 18 (26) | 7 (19) | 0.47 |
| Any Cancer (solid or liquid tumor) | 93 (27) | 37 (35) | 0.09 | 27 (39) | 10 (28) | 0.27 |
Presented as Number (%) unless otherwise specified
COPD = chronic obstructive pulmonary diseases, HIV = human immunodeficiency virus, SD = Standard Deviation.
There was no statistically significant difference (P > 0.20) across groups for stroke (ischemic or hemorrhagic), solid organ transplant, history of coronary artery disease, or acute coronary syndrome.
Table 2.
Severity of Illness, Process of Care Measures and Clinical Outcomes According to Mechanical Ventilation Status among 457 Patients with ARDS
| All ARDS N=457
|
P-value | Initially Non-Intubated N=106
|
P-value | |||
|---|---|---|---|---|---|---|
| Early-Intubation N=351 | Initially Non-Intubated N=106 | Never-Intubated N=70 | Late-Intubated N=36 | |||
| Severity of Illness | ||||||
| Primary ARDS risk factor | 0.03 | 0.65 | ||||
| Sepsis | 147 (42) | 43 (41) | 27 (39) | 16 (44) | ||
| Pneumonia | 95 (27) | 34 (32) | 23 (33) | 11 (31) | ||
| Aspiration | 74 (21) | 11 (10) | 9 (13) | 2 (6) | ||
| Other | 35 (10) | 18 (17) | 11 (16) | 7 (19) | ||
| Respiratory rate (breaths per minute) | 31 ± 9 | 33 ± 8 | 0.01 | 32 ± 8 | 34 ± 7 | 0.27 |
| PaO2/FiO2 a, Mean ± SD | 146 ± 84 | 181 ± 86 | 0.006 | 180 ± 87 | 182 ± 87 | 0.93 |
| SpO2/FiO2b, Mean ± SD | 160 ± 62 | 211 ± 76 | <0.001 | 212 ± 79 | 212 ± 71 | 0.99 |
| Shock (circulatory failure) | 261 (74) | 50 (47) | <0.001 | 34 (49) | 16 (44) | 0.69 |
| Coagulation failure | 74 (21) | 30 (28) | 0.12 | 20 (29) | 10 (28) | 0.93 |
| Renal failure | 111 (32) | 30 (28) | 0.52 | 20 (29) | 10 (28) | 0.93 |
| Hepatic failure | 74 (21) | 14 (13) | 0.07 | 8 (11) | 6 (17) | 0.45 |
| APACHE II, Mean ± SD | 31 ± 7 | 22 ± 6 | <0.001 | 22 ± 6 | 23 ± 7 | 0.57 |
| Process Measures | ||||||
| Time from admission to ICU in days, Median (IQR) | 0 (0–3) | 1 (0–4) | 0.45 | 1 (0–4) | 1 (0–2.5) | 0.89 |
| NIPPV on initial day of lung injury | 2 (1) | 20 (19) | <0.001 | 13 (19) | 7 (19) | 0.91 |
| Fluid Balance in liters on enrollment, Median (IQR) | 2.8 (1.0 to 5.6) | 1.2 (−0.4 to 2.7) | <0.001 | 1.2 (−0.2 to 3.2) | 1.0 (−0.6 to 2.3) | 0.66 |
| Clinical Outcomes | ||||||
| Death at 60 days | 128 (36) | 38 (36) | 0.91 | 18 (26) | 20 (56) | 0.002 |
| Died in the hospital | 104 (30) | 28 (26) | 0.52 | 10 (14) | 18 (50) | <0.001 |
| Ventilator-free days, Median (IQR) | 16 (0 to 23) | 24 (8 to 28) | <0.001 | 28 (23 to 23) | 7 (1 to 20) | <0.001 |
| ICU days in hospital survivors, Median (IQR)c | 9 (6 to 16) | 6 (3 to 10) | <0.001 | 4 (3 to 7) | 11.5 (9 to 17) | <0.001 |
| Days of MV in hospital survivors, Median (IQR)c | 6 (3 to 12) | 0 (0 to 4) | <0.001 | 0 (0 to 0) | 8 (4 to 15) | <0.001 |
Presented as Number (%) unless otherwise indicated
Abbreviations: APACHE = acute physiology and chronic health evaluation, NIPPV = Non-invasive positive pressure ventilation, IQR = interquartile range, MV = mechanical ventilation, SD = standard deviation,
Available in 339 patients, 283 early-intubation, 56 initially non-intubated
Available in 420 patients, 327 early-intubation, 93 initially non-intubated
247 Early-intubation and 78 initially non-intubated patients survived to discharge; Of initially non-intubated patients, 60 never-intubated, 18 late-intubation
Statistical analysis
For bivariate analysis, the Wilcoxon test and t-test were used for continuous variables as appropriate, and the χ2 test was used for categorical variables. Kaplan-Meier survival plots demonstrate the time from admission to 60 days and two years follow-up.
Two multivariate models were used to evaluate the effect of potential confounders on the association between intubation status and mortality at 60 days, 1 year and 2 years follow-up. Both regression models incorporated baseline demographic, comorbidities and severity of illness measures (Tables 1 and 2) that varied according to intubation status with a P-value < 0.20. Variables included in the models were sex, source of admission, alcohol abuse by history, current smoker, established diagnosis of COPD, HIV, cirrhosis, leukemia or stem cell transplant, any cancer diagnosis, ARDS risk factor, respiratory rate, severity of hypoxemia (according to PaO2/FiO2 or SpO2/FiO2), presence of shock, hepatic failure, APACHE II, NIPPV use and fluid balance. First, a cox proportional hazards backward selection model approach was utilized. Second, a propensity score was generated to estimate the causal effects of late endotracheal intubation on mortality within the initially non-ventilated group. Propensity score quintiles were then included in a Cox proportional hazards regression model with likelihood of late intubation as the dependent variable. Goodness of fit and discrimination of the model were assessed using the Hosmer-Lemeshow test and C-statistic, respectively.
A sensitivity analysis was performed reclassifying patients receiving NIPPV on the day of ARDS diagnosis into the early-intubation group, since NIPPV is included in the current Berlin definition of mild ARDS and has been included in some other epidemiologic studies of ARDS to date.(7, 15, 37–43)
The analyses were performed using STATA version 12 (STATA Corp, College Station, TX). Statistical significance was defined as a two-tailed P < 0.05 for all analyses.
RESULTS
Among 457 patients with evidence of ARDS, 23% (N=106) were not intubated and mechanically ventilated (initially non-intubated) on day 1 and 77% were intubated and mechanically ventilated (early-intubation) on day 1 (Figure 2). Of the 106 initially non-intubated patients, only 36 (34% of initially non-intubated) progressed to require intubation (late-intubation) in the subsequent follow-up period, whereas 70 patients (66% of initially non-intubated) did not require intubation (never-intubated) during the follow-up period.
Figure 2.
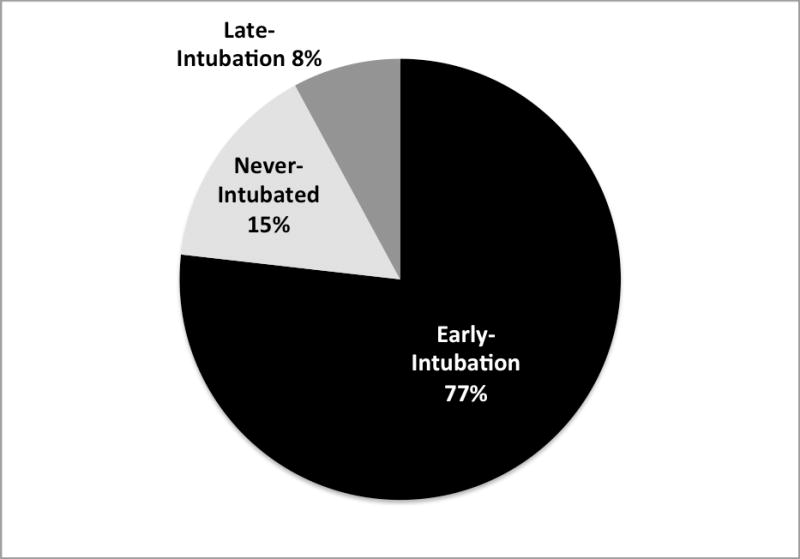
Intubation group (Early-intubation, never-intubated, late-intubation) among 457 patients with ARDS
Initially non-intubated patients differed significantly from the early-intubation group (Tables 1 and 2, columns 2–4). Compared to the early-intubation group, patients who were initially non-intubated at the time of ARDS diagnosis were more likely to be admitted from the emergency department and transferred from the floor (P-values ≤ 0.001). Initially non-intubated patients were less likely to have a known history of alcohol abuse (6% versus 16%, P = 0.006) but were more likely to have an underlying hematologic malignancy (24% versus 7%, P < 0.001). The severity of illness was lower in initially non-intubated patients compared to early-intubation patients (Table 2) with lower mean APACHE II score (22 ± 6 versus 31 ± 7, P < 0.001), less severe hypoxemia (PaO2:FiO2 181 ± 86 mmHg versus 146 ± 84 mmHg, P = 0.006; SpO2:FiO2 211 ± 76 versus 160 ± 62, P < 0.001), and lower rates of shock (47% versus 74%, P < 0.001). Initially non-intubated patients were more likely to be treated with NIPPV (9% versus 1%, P < 0.001) on the day of meeting ARDS criteria, and respiratory rates were increased in initially non-intubated patients compared to those with early-intubation (P = 0.01). Fluid balance in both groups was positive measured from the 24 hours prior to enrollment but was lower in the initially non-intubated group compared to the early-intubation group (+1.2 liters versus 2.8 liters, P < 0.001).
Among the 106 initially non-intubated patients, there were few demographic or initial clinical differences between the minority who progressed to require intubation (late-intubation) and the majority who did not (never-intubated) (Tables 1 and 2, columns 5–7). There was no difference between the groups in the proportion of patients treated with NIPPV (19% in both never-intubated [N=13 of 70] and late-intubation [N=7 of 36] groups, P = 0.91). The late-intubation group was more likely to have a history of cirrhosis (11% versus 1%, P = 0.03) compared to patients who did not progress to require endotracheal intubation for lung injury. However, other demographic and presenting clinical characteristics including age, sex, race, source of admission, serious comorbidities and severity of illness measures were similar between groups.
Mechanical ventilation status and clinical outcomes
Mortality at 60 days was the same in initially non-intubated patients compared to early-intubation patients (Table 2, 36% in each group, P = 0.91). Patients in the early-intubation group had increased overall respiratory failure as measured by fewer VFD, increased number of ICU days and increased days of mechanical ventilation (P < 0.001 for all comparisons, Table 2), compared to the initially non-intubated group.
After classifying patients according to intubation status over the 4-day follow-up period, patients in the late-intubation subgroup had significantly increased mortality at 60 days compared to both the never-intubated (56% versus 26%, P = 0.002) and early-intubation groups (56% vs. 36%, P = 0.03) (Table 3). The majority (N = 27) of the late-intubation group underwent intubation on day 2 of follow-up (Figure 3). An additional 9 patients underwent intubation on days 3 and 4 after meeting ARDS criteria. Mortality at 60 days was similarly elevated in the Day 2 and Day 3–4 late-intubation subgroups (Figure 3). Differences in mortality across intubation groups persisted at 60 days (P = 0.004) and at both one- (P = 0.01) and two-year (P = 0.02) follow-up (Figures 4a and b).
Table 3.
Clinical Outcomes in Three Intubation Groups
| N | Early-intubation 351 |
Never-Intubated 70 |
Late-Intubation 36 |
|---|---|---|---|
| Death at 60 days, n (%) | 128 (36) | 18 (26) | 20 (56)a,b |
| Died in the hospital, n (%) | 104 (30) | 10 (14)a | 18 (50)a,b |
| Ventilator-free days, Median (IQR) | 16 (0 to 23) | 28 (23 to 28)a | 7 (1 to 20)b |
| ICU days, Median (IQR)c | 9 (6 to 16) | 4 (3 to 7)a | 11.5 (9 to 17)b |
| Days of MV, Median (IQR)c | 6 (3 to 12) | 0 (0 to 0)a | 8 (4 to 15)b |
P < 0.05 versus early-intubation
P < 0.05 versus never-intubated
Among survivors to hospital discharge: 247 early-intubation and 78 initially non-intubated patients. Of initially non-intubated patients, 60 were never-intubated and 18 underwent late-intubation
Figure 3.
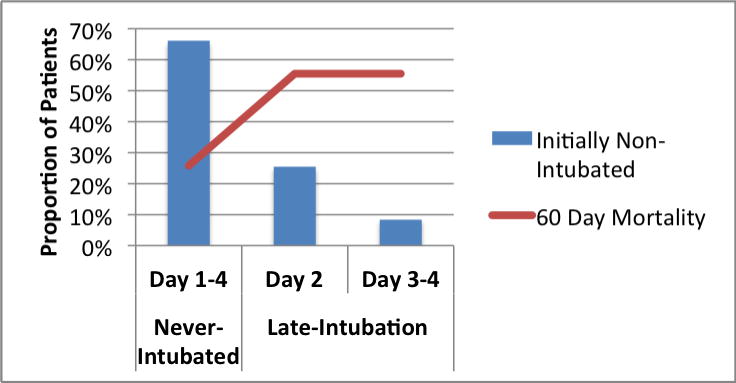
Timing of intubation in 106 initially non-intubated patients with ARDS
Figure 4.
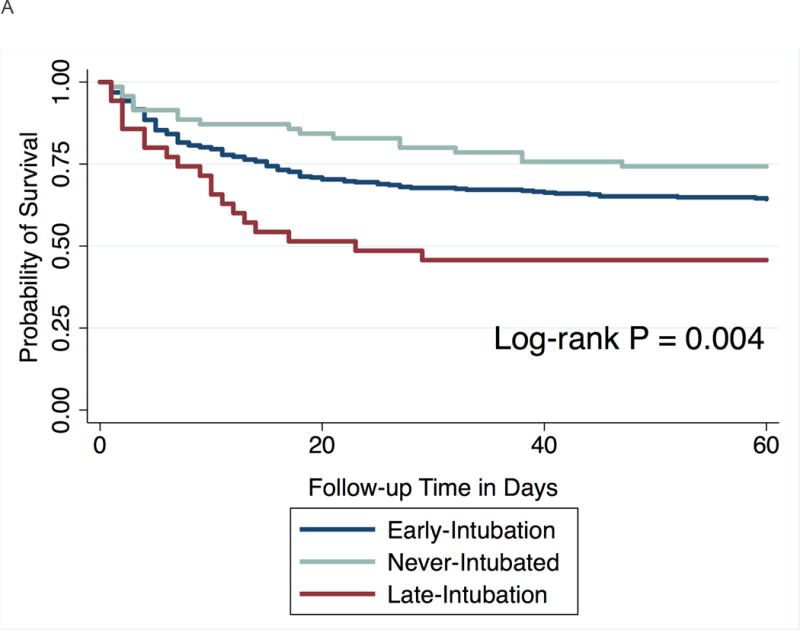
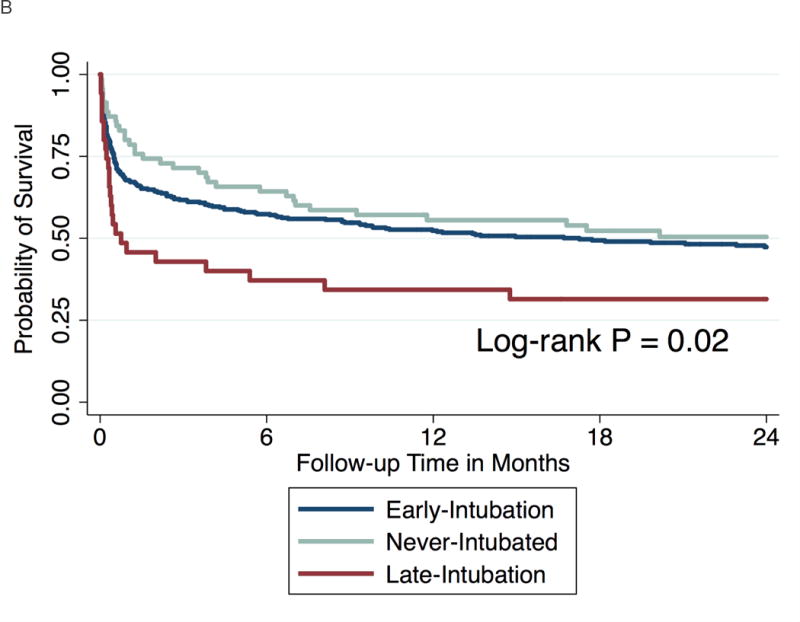
Kaplan-Meier curve showing probability of survival at follow-up.
a. At 60 days
b. At two years follow-up
The late-intubation subgroup also had significantly fewer VFDs, more days requiring mechanical ventilation (MV) and increased ICU days in survivors to hospital discharge compared to the never-intubated group (Table 3, all P-values < 0.05). Although there was a trend toward lower VFD and increased ICU and MV days in the late-intubation group compared to the early-intubation patients, these differences were not statistically significant (Table 3).
Multivariate Analyses
Using a Cox proportional hazards, backward selection model (final variables selected for 60-day follow-up are specified in table 4), the adjusted risk of death at 60 days was 2.37-times higher in the late-intubation group compared to early-intubation (95% CI 1.32 – 4.24, P = 0.004). In contrast, there was no significant difference in mortality in the never-intubated and early-intubation groups at 60 days in adjusted analysis. Results were similar at both 1- and 2-year follow-up (data not shown).
Table 4.
Risk of Death at 60 days compared to Early-intubation referent*
| Unadjusted | Multivariate Adjusteda | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-Value | HR | 95% CI | P-Value | |
| Death at 60 days | ||||||
| Never-intubated | 0.64 | 0.39 – 1.05 | 0.08 | 0.76 | 0.42 – 1.38 | 0.37 |
| Late-intubation | 1.81 | 1.13 – 2.90 | 0.01 | 2.37 | 1.32 – 4.24 | 0.004 |
Abbreviations: HR = Hazard ratio
Results at 1 and 2 years similar
Backward section including variables associated with intubation status, P < 0.20. Final selected variables: alcohol abuse by history, admission from the operating room, cirrhosis, leukemia or stem cell transplant, any cancer, hepatic failure, APACHE II, NIPPV use, ARDS risk factor, and fluid balance at time of enrollment
Follow-up 90% at one year (411/457), and 81% at two years (369/457).
A propensity score to account for baseline covariates that differed according to intubation status was limited to 106 initially non-intubated patients. Model fit of the Cox-proportional hazards regression model adjusting for propensity quintile was adequate (Goodness-of-fit, P = 0.81), and the c-statistic was 0.77. The distribution of propensity scores was similar in the never-intubated and late-intubation groups. After adjustment for propensity quintile, late-intubation was associated with a 3.53-fold increased risk of death at 60 days compared to never-intubated patients (95% CI 1.70 – 7.34, P = 0.001, Table 5). At one and two years follow-up the late-intubation group remained at 2.5-fold increased risk of death compared to patients that did not require intubation.
Table 5.
Risk of Death in the late-intubation group compared to never-intubated referent
| Unadjusted | Propensity Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-Value | HR | 95% CI | P-Value | |
| Death at 60 days | 2.86 | 1.51 – 5.43 | 0.001 | 3.53 | 1.70 – 7.34 | 0.001 |
| Death at 1 year | 2.15 | 1.26 – 3.67 | 0.005 | 2.54 | 1.37 – 4.69 | 0.003 |
| Death at 2 years | 2.06 | 1.22 – 3.46 | 0.006 | 2.50 | 1.36 – 4.60 | 0.003 |
Abbreviations: HR = Hazard ratio
Reclassifying Patients Treated with NIPPV as “Intubated”
Among patients who were non-intubated on the day of meeting ARDS criteria, NIPPV was used for an equal proportion of the never-intubated and late-intubated groups (19% in each group, Table 2). Mortality at 60 days among patients treated with NIPPV was high at 55%. However, there was no evidence that NIPPV modified the association between intubation and mortality (Test of interaction P = 0.40). In a sensitivity analysis reclassifying NIPPV as part of the early-intubation group (since noninvasive positive pressure ventilation is included in the Berlin definition for ARDS), the differences in mortality between intubation groups remained similar (Figure 1, supplementary appendix).
DISCUSSION
The primary findings of this study can be summarized as follows. First, in a multi-ICU tertiary care center prospective cohort, 23% of patients otherwise meeting criteria for ARDS, as defined as acute onset of hypoxemia and non-cardiogenic pulmonary edema, did not require intubation and mechanical ventilation on the day of meeting ARDS criteria. Second, only a minority of these patients (34% of initially non-intubated) later progressed to require endotracheal intubation and mechanical ventilation, most within the subsequent 1–2 days. This subset of patients that underwent late-intubation had markedly higher mortality rates compared to both patients who were intubated early and patients that never progressed to require intubation. This observation withstood adjustment for comorbidities and severity of illness on the day of ARDS diagnosis. These findings support and extend upon the findings of a prior pediatric study(20) and adult studies(21–25) indicating that it is feasible and important to identify non-intubated patients with ARDS, in part to facilitate earlier treatment and hopefully improve outcomes.(19)
This study enriches the understanding of the epidemiology of ARDS and complements the few existing studies of non-intubated patients with ARDS.(21, 24, 25) Cely and colleagues found that only 57% of patients meeting AECC criteria for ALI/ARDS in a Veteran Affairs medical center were initially invasively mechanically ventilated in the ICU.(21) Of the remaining 43%, there were 26% who were non-mechanically ventilated in the ICU and 17% who were never mechanically ventilated nor admitted to the ICU. Similarly, Quartin and colleagues and Ferguson et al identified acute lung injury among non-intubated patients in non-ICU wards.(24, 25). Our study demonstrates that ARDS is prevalent among ICU patients prior to developing respiratory failure severe enough for intubation and in patients never requiring intubation.
Our research group has previously studied patients presenting to the emergency department with bilateral opacities on chest radiograph prior to the need for endotracheal intubation in order to establish a definition of “early” acute lung injury. (22, 23) The goal of these studies was to identify clinical predictors of progression to ARDS requiring positive pressure ventilation (via endotracheal tube or face mask). While these prior studies focused on less acutely ill patients, the majority of whom were admitted to non-ICU beds, and excluded all patients meeting consensus criteria for ARDS receiving positive pressure mechanical ventilation on presentation, they identified a similar proportion of patients with ARDS without fulminant respiratory failure who went on to require intubation as in the current study (25–33% versus 34%).
In the current study, we identified increased mortality in the late-intubation subgroup that was not explained by demographics, comorbidities, initial severity of illness or propensity to receive endotracheal intubation. No clinical or demographic factor clearly predicted the clinical deterioration for these patients. In contrast, patients with early-intubation were markedly sicker at the time of ARDS diagnosis with increased organ dysfunction, shock, and higher APACHE II scores compared to non-intubated patients, including the late-intubation subgroup. Moreover, the increased risk of death for the late-intubation group persisted at both one and two years of follow-up and withstood reclassification of patients receiving NIPPV to the early-intubation group; thus, there was no evidence that delay of endotracheal intubation through the use of NIPPV mediated the increased mortality observed in late-intubation subgroup. One possible explanation for increased risk of death in the late-intubation subgroup includes the higher proportion of patients with malignancy in the initially non-intubated group compared to the early intubation patients; however, the proportion of these patients did not significantly differ between the never-intubated and late-intubation subgroups, and, in fact, there was a trend to higher prevalence of malignancy among the never-intubated group, suggesting that delay in intubation due to malignancy was not a likely explanation.
These results have implications for clinical practice in terms of providing new epidemiologic data on the clinical manifestations and outcomes in ARDS and also for timing of patient selection in future studies. Because current definitions of ARDS exclude patients not requiring positive pressure respiratory support, both researchers and clinicians may miss opportunities to diagnose and treat patients with high morbidity and mortality earlier in the course of illness. Further, with the growing data supporting the therapeutic value of high-flow nasal oxygen over NIPPV in acute hypoxemic respiratory failure,(44) the proportion of ARDS patients that never require intubation or require intubation and mechanical ventilation later in the course of illness is likely to grow. Yet there are no clear clinical classifications for these patients – only after they were treated with NIPPV or intubated did these patients meet the classical definition of Berlin ARDS.(7) Further study in larger cohorts is warranted to confirm the increased mortality observed particularly in the late-intubation group. The current study cannot assess causality, and clinical factors predicting late-intubation were not identified. One possible contributor to worse outcomes in the late-intubation group may have been delayed intubation. However, at the time of diagnosis of ARDS, these patients did not appear to be significantly different from the “never intubated” group; therefore, this study cannot provide insight as what early signs may have predicted decline in these patients. More work must be done to identify patients likely to decline before they require intubation in order to eventually test the hypothesis that early intubation in a higher risk groups could improve outcomes. In addition, future studies must incorporate alternative therapies such as high flow nasal oxygen, which are likely to reduce the need for positive pressure ventilation and potentially reduce ARDS mortality.(44, 45)
Strengths of the current study include its prospective design, large study sample, detailed phenotyping of clinical characteristics and severity of illness, and long-term follow-up. Importantly, patients with a Do Not Intubate order were excluded from this study so that differences in goals of care would not bias the results. However, some limitations warrant discussion. First, the study was carried out at a single, tertiary care site. However, the study included a large, multi-disciplinary medical and surgical subcohort of ARDS patients derived from a broad range of critically ill patients, which is likely to improve generalizability overall. Further, this study is unique in that it included patients that were non-intubated at the time of ARDS diagnosis. Second, while several recent studies have reported that non-intubated patients represent a substantial fraction of the ARDS population in both adults and children,(21, 24, 25) there are challenges in defining the severity of hypoxemia in this population. The FiO2 is more difficult to measure accurately, and thus the PaO2/FiO2 (and likely the SpO2/FiO2) are less reliable at low FiO2 due to shunting.(46) Further, PEEP ≥10 cm H2O has been associated with improved consistency in the measurement of hypoxemia,(47) and half of the mechanically ventilated patients and all of the non-mechanically ventilated patients in our study had either lower PEEP levels or no supplemental PEEP, potentially leading to an overestimation of the severity of hypoxemia in these patients. However, the purpose of this study was to study the epidemiology and clinical outcomes in patients with clinical and radiographic evidence of ARDS and some degree of arterial hypoxemia prior to mechanical ventilation. This approach enabled us to identify differences in clinical outcomes according to the timing of mechanical ventilation requirement. Third, we do not have detailed data on indications for intubation, exact timing of intubation or detailed data on ventilator management in this cohort. These are details that will be critical to obtain in future studies to better understand the epidemiology of ARDS in initially non-intubated patients. Finally, this study does not include non-ICU patients, and further study of clinical outcomes in this population is necessary.
CONCLUSIONS
The results of this study demonstrate that a large subset of patients with ARDS are never intubated and those that are intubated later in the course of illness have poor clinical outcomes. Current definitions of ARDS do not include most of these non-intubated patients with ARDS, and both researchers and clinicians may miss opportunities to diagnose and treat these patients earlier in the course of illness. Consensus definitions and further prospective epidemiologic, treatment and biology studies are necessary to identify high-risk non-intubated patients with ARDS. These patients may represent an ideal target for novel therapies.
Supplementary Material
Supplementary Figure 1. Kaplan-Meier curve showing probability of survival at 60 days after reclassifying patients undergoing NIPPV with the “early-intubation” group
Acknowledgments
We thank all the patients who participated in the study and the research staff who assisted with the study. None of the supporting funding sources had any role in the collection of data, interpretation of results, or preparation of this manuscript.
Source of funding: Dr. Ware has served on a medical advisory board for Glaxo Smith Kline and as a consultant for Abbot. Dr. Calfee has served on medical advisory boards for Cerus Corp and GlaxoSmithKline. Dr. Matthay has served on medical advisory boards for Cerus Corporation, GlaxoSmithKline, and Roche Genentec. Drs. Calfee and Matthay have also received grant support from GlaxoSmithKline. At the time the research was conducted Dr. Kangelaris was supported by the Society of Hospital Medicine Young Researchers Award, the NIH National Center for Advancing Translational Sciences through UCSF-CTSI KL2 TR000143, and NHLBI 1K23HL116800–01. Dr. Calfee was supported by HL110969. Dr Ware was supported by NHLBI HL112656, and HL103836. Dr. Matthay was supported by NHLBI R37 HL51856. Dr. Janz was supported by NIH T32 HL087738.
Copyright form disclosures: Dr. Kangelaris received support for article research from the National Institutes of Health (NIH), UCSF-CTSI KL2 TR000143, and NHLBI 1K23HL116800–01. Her institution received grant support from the NIH, NHLBI, and Society of Hospital Medicine grant. Dr. Ware consulted for Glaxo Smith Kline and Abbott and received support for article research from the NIH. Her institution received grant support from the NIH and the American Heart Association. Dr. Zhuo received support for article research from the NIH. Dr. Matthay received funding from Quark Pharmaceuticals and from Cerus (ARDS consult – money paid to Dr. Matthay), received support for article research from the NIH, and received funding from GSK (consultant for ARDS and grant for studies of sepsis, grant to UCSF his institution). His institution received funding from Roche-Genentec (Chair of DSMB for Asthma trials). Dr. Calfee received support for travel from Boehringer Ingelheim (travel for meeting related to potential research grant); received support for article research from the NIH). Her institution received grant support from the NIH and from Glaxo Smith Kline and consulted for Glaxo Smith Kline and Cerus (advisory boards).
Footnotes
Conflicts of Interest
No author (KNK, LBW, CW, DRJ, HZ, MAM, CSC) reports a conflict of interest.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bell RC, Coalson JJ, Smith JD, et al. Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med. 1983;99(3):293–298. doi: 10.7326/0003-4819-99-3-293. [DOI] [PubMed] [Google Scholar]
- 3.Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 4.Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 5.Sloane PJ, Gee MH, Gottlieb JE, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146(2):419–426. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165(4):443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 10.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 11.Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31(6):1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 12.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159(6):1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 13.Matthay MA, Brower RG, Carson S, et al. Randomized, Placebo-Controlled Clinical Trial of an Aerosolized Beta-2 Agonist for Treatment of Acute Lung Injury. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roupie E, Lepage E, Wysocki M, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Societe de Reanimation de Langue Francaise. Intensive Care Med. 1999;25(9):920–929. doi: 10.1007/s001340050983. [DOI] [PubMed] [Google Scholar]
- 15.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 17.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 18.Levitt JE, Matthay MA. The utility of clinical predictors of acute lung injury: towards prevention and earlier recognition. Expert Rev Respir Med. 2010;4(6):785–797. doi: 10.1586/ers.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NHLBI Clinical Trials Research Network for the Prevention and Treatment of Acute Lung Injury (PETAL Network) 2014 [cited 2014 August 21] Available from: http://petalnet.org.
- 20.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 21.Cely CM, Rojas JT, Maldonado DA, et al. Use of intensive care, mechanical ventilation, both, or neither by patients with acute lung injury. Crit Care Med. 2010;38(4):1126–1134. doi: 10.1097/CCM.0b013e3181d56fae. [DOI] [PubMed] [Google Scholar]
- 22.Levitt JE, Bedi H, Calfee CS, et al. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitt JE, Calfee CS, Goldstein BA, et al. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit Care Med. 2013;41(8):1929–1937. doi: 10.1097/CCM.0b013e31828a3d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quartin AA, Campos MA, Maldonado DA, et al. Acute lung injury outside of the ICU: incidence in respiratory isolation on a general ward. Chest. 2009;135(2):261–268. doi: 10.1378/chest.08-0280. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kangelaris KN, Calfee CS, May AK, et al. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care. 2014;4(1):4. doi: 10.1186/2110-5820-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014 doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 30.Bazuaye EA, Stone TN, Corris PA, et al. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47(8):609–611. doi: 10.1136/thx.47.8.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604–609. [PubMed] [Google Scholar]
- 32.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112(1):164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 35.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 36.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 37.Rocker GM, Mackenzie MG, Williams B, et al. Noninvasive positive pressure ventilation: successful outcome in patients with acute lung injury/ARDS. Chest. 1999;115(1):173–177. doi: 10.1378/chest.115.1.173. [DOI] [PubMed] [Google Scholar]
- 38.Auriant I, Jallot A, Herve P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164(7):1231–1235. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 39.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 40.Rana S, Jenad H, Gay PC, et al. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care. 2006;10(3):R79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive-pressure ventilation in acute respiratory failure outside clinical trials: experience at the Massachusetts General Hospital. Crit Care Med. 2008;36(2):441–447. doi: 10.1097/01.CCM.0000300084.67277.90. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010;55(12):1653–1660. [PubMed] [Google Scholar]
- 43.Thille AW, Contou D, Fragnoli C, et al. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013;17(6):R269. doi: 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frat JP, Thille AW, Mercat A, et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 45.Matthay MA. Saving lives with high-flow nasal oxygen. N Engl J Med. 2015;372(23):2225–2226. doi: 10.1056/NEJMe1504852. [DOI] [PubMed] [Google Scholar]
- 46.Gowda MS, Klocke RA. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med. 1997;25(1):41–45. doi: 10.1097/00003246-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Villar J, Perez-Mendez L, Lopez J, et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176(8):795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan-Meier curve showing probability of survival at 60 days after reclassifying patients undergoing NIPPV with the “early-intubation” group


