Fig 4.
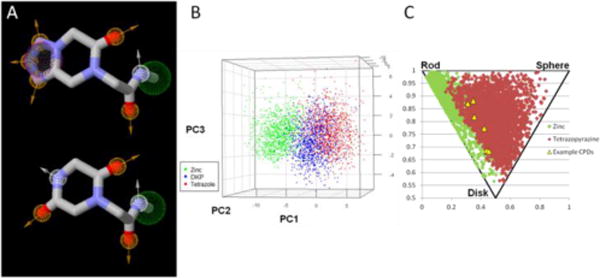
(a) Pharmacophore point comparison of our tetrazole-fuzed keto-piperazines (top) and their diketopiperazine counterparts, synthesized via a variation of the Ugi reaction (green ball = hydrophobic, orange ball = hydrogen bond acceptor, white ball = hydrogen bond donor) ; (b) 3D PCA of 1000 randomly selected tetrazoles (red), DKPs (blue), and Zinc compounds (green) (c) PMI plot depicting 1000 randomly selected tetrazole compounds (red) overlapped with the PMI plot of 1000 randomly selected compounds from the ZINC database (green). Six of the compounds from the paper have also been plotted (yellow triangles).
