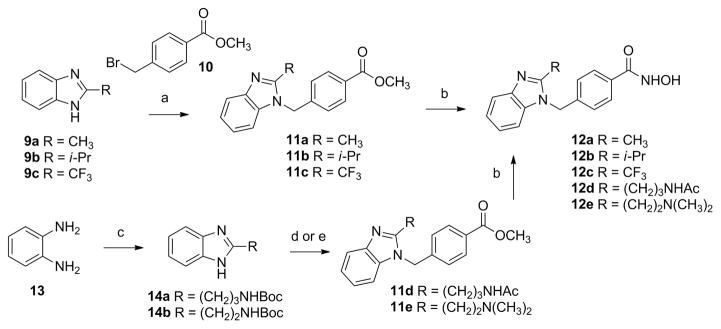
Scheme 1. Synthesis of Benzimidazole-Based Analoguesa.
aReagents and conditions: (a) K2CO3, DMF, 80 °C; (b) NaOH, 50 wt % aq. NH2OH, THF/MeOH (1:1), 0 °C to rt; (c) (i) EDCI, Et3N, DCM, N-Boc-GABA-OH or N-Boc-βAla-OH, 0 °C to rt; (ii) AcOH, MeOH, 65 °C; (d) (i) 10, K2CO3, DMF, 80 °C; (ii) HCl, acetone, 50 °C; (iii) Ac2O, pyridine, DMAP, DCM, 0 °C to rt, for the synthesis of 11d; (e) (i) 10, K2CO3, DMF, 80 °C; (ii) HCl, acetone, rt; (iii) aq. CH2O, NaCNBH3, MeOH, rt, for the synthesis of 11e.