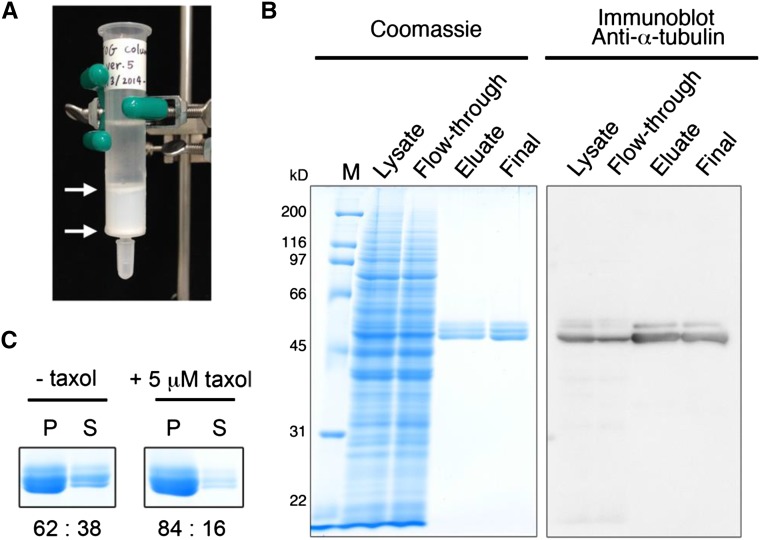
Figure 1.
Affinity purification of Arabidopsis tubulin using the TOG column. A, A gravity-flow TOG column. The GST-TOG1/2-coupled resin (2 mL) is sandwiched between the upper and lower porous plastic filters (arrows) and packed in a 15-mm-diameter column. B, Purification of tubulin from Arabidopsis MM2d cultured cells. Protein samples taken from different purification steps were separated by SDS-PAGE and analyzed by Coomassie Blue staining (left) and immunoblotting with anti-α-tubulin antibody (right). M, Molecular mass marker; Lysate, 5 μL of crude cell extract containing 27 μg of proteins; Flow-through, 5 μL of the flow-through fraction that did not bind to the TOG column; Eluate, 10 μL of the TOG column eluate; Final, 1 μg of the desalted and concentrated tubulin. C, MT sedimentation assay. Purified MM2d tubulin (20 μm) was assembled into MTs in the absence (left) or presence of 5 μm taxol (right). Coomassie Blue-stained tubulin bands in the pellet (P) and supernatant (S) fractions represent polymerized MTs and tubulin, respectively. Proteins were quantified by densitometric analysis, and ratios (%) of the pellet to supernatant fractions are indicated.