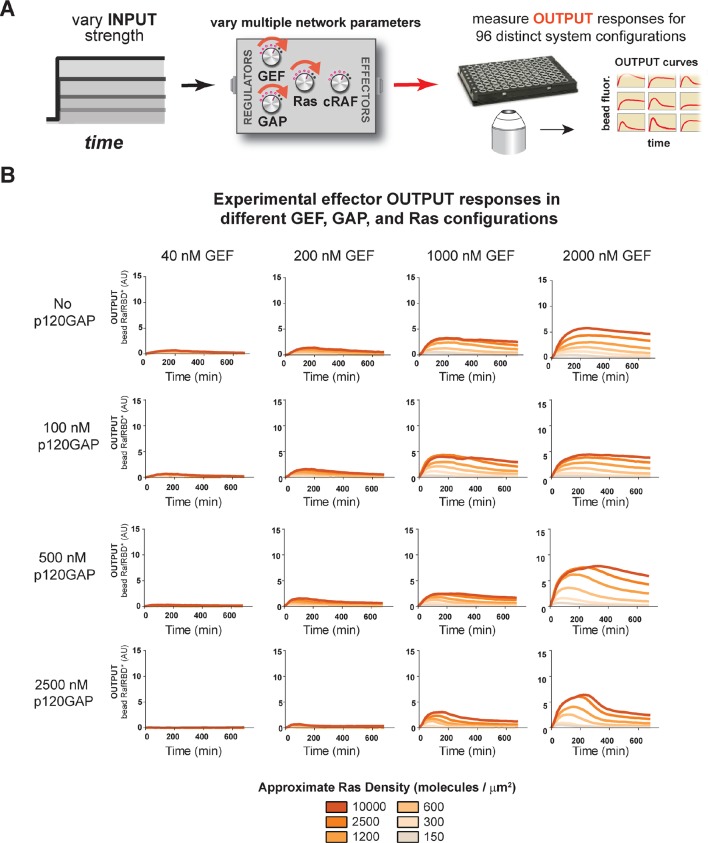
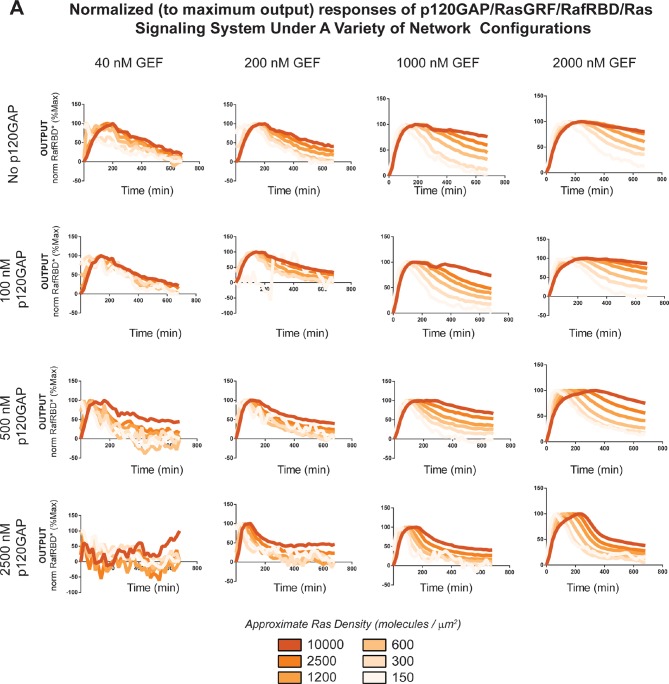
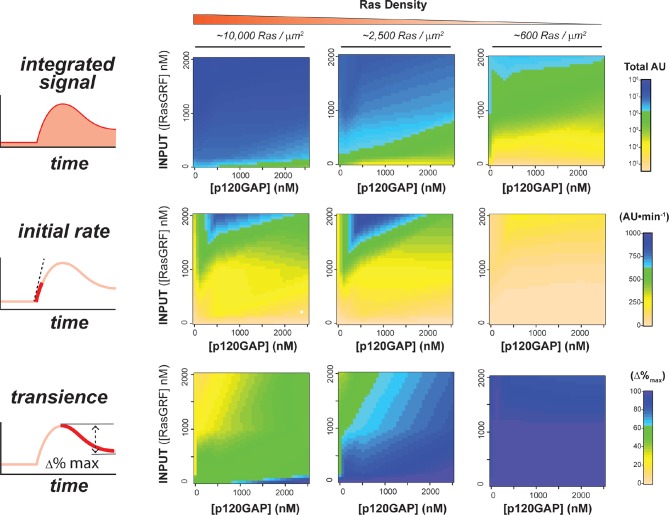
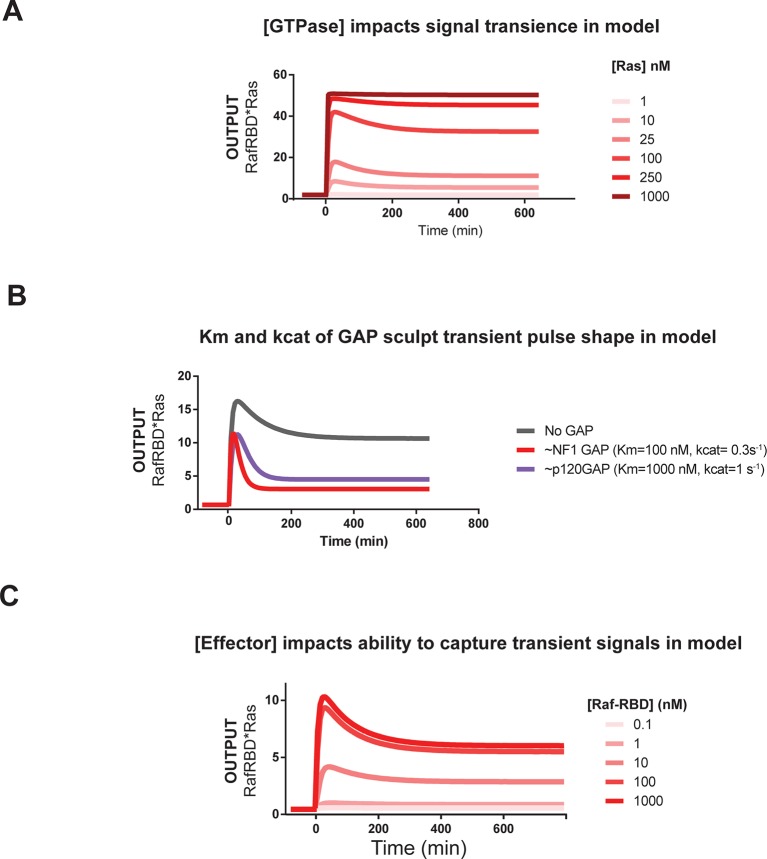
(A) Output of Kintek simulation using a three-state GTPase model with competition between GAP and effectors as described in the main-text 'Materials and methods', in which the Ras density (i.e. concentration in this model) is varied over four orders of magnitude as indicated. Initial conditions were 50 nM effector, 1 μM GEF, no GAP, and 'infinite' nucleotide (100000 nM). The model recovers the observation that at low densities, more transient behavior is observed than at high Ras densities, which show a more associative response. (B) Output of Kintek simulation using a three-state GTPase model with competition between GAP and effectors as described in the main-text 'Materials and methods', in which GAP parameter choices that resemble the NF1-GAP (koff = 0.01 s-1, kcat = 0.1 s-1) or p120GAP (koff = 0.25 s-1, kcat = 0.4 s-1) are used. Initial conditions were 50 nM effector, 10 nM Ras, 1 μM GEF, 1 μM GAP, and 'infinite' nucleotide (100000 nM). This model recovers the observation that differences in Km and kcat can result in equivalent amounts of NF1gap and p120GAP producing different transient behaviors in the system output. (C) Output of Kintek simulation using a three-state GTPase model with competition between GAP and effectors as described in the main-text Materials and methods, in which effector concentrationsare varied over 5 orders of magnitude. Initial conditions were 10 nM Ras, 1 μM GEF, no GAP, and 'infinite' nucleotide (100000 nM). This model recovers the observation that higher effector concentrations allow more transient features of the time-varying GTPase signal to be captured in the system output. GAP, GTPase-activating protein; GEF, guanine exchange factor.