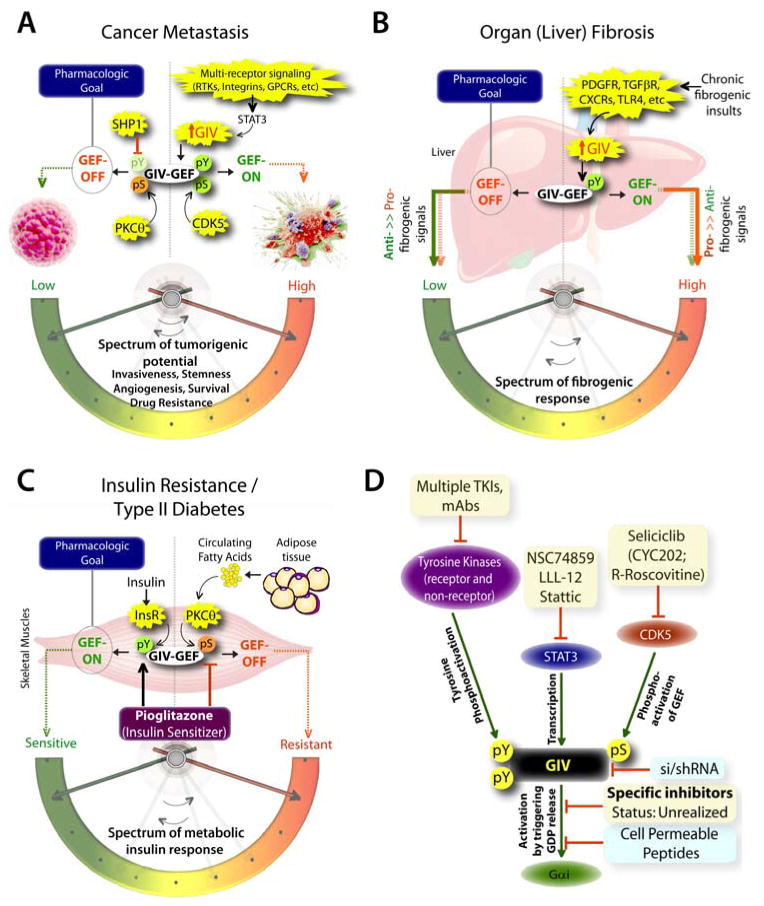
Figure 3. The untapped pharmacologic potential of GIV in chronic, multi-receptor driven disease states.
A) Schematic summarizing our and others’ work on how GIV modulates several parameters, e.g., invasiveness, stemness, angiogenesis, survival, and drug resistance, all of which together constitute the tumorigenic potential of cancer cells. Each of these parameters, that can be triggered by diverse classes of oncogenic receptors, are driven more efficiently and consequently, the tumorigenic potential is highest when GIV expression is elevated and its GEF function is ‘ON’ [summarized in (8, 85)]. GIV levels are transcriptionally upregulated in invasive cancer cells during cancer progression via the transcription factor, STAT3 (63)., whereas its GEF function is turned ‘ON’ via a single phosphoevent that is triggered by CDK5 (50). Because GIV serves as molecular rheostat, i.e., signaling intensity via GIV’s GEF function is closely related to the two variables, i.e., number of GIV molecules in cells and whether their GEF function is ‘ON’, it is expected that by altering both variables one can alter the tumorigenic potential of cancer cells from low to high. Thus, the pharmacologic goal to halt cancer progression is to reduce the copies of GIV (by tumor-targeted si/shRNA) and/or to selectively turn ‘OFF’ GIV’s GEF function (using small molecule inhibitors). Antagonistic pathways, such as SHP1 phosphatase (90) and PKCθ kinase (23), which terminate tyrosine-based and G protein-based signals, respectively, may also be potentiated to accomplish a similar goal. B) Schematic summarizing our work (76) on how GIV regulates several parameters, e.g., proliferation, chemotaxis, survival, metalloprotease and collagen production, all of which together constitute the fibrogenic potential of myofibroblasts in response to chronic injury/inflammation. Each of these parameters, that can be triggered by diverse classes of fibrogenic receptors, are driven more efficiently and consequently, the fibrogenic potential is highest when GIV expression is elevated and its GEF function is ‘ON’. Upon chronic fibrogenic injury, GIV levels are transcriptionally upregulated and GIV’s GEF function is turned ‘ON’ in myofibroblasts. When GIV’s GEF function is ‘ON’, pro-fibrogenic (red arrows) signals are potentiated, whereas anti-fibrogenic (green arrows) signals are downregulated; the opposite occurs when GIV’s GEF function is turned ‘OFF’. Thus, the pharmacologic goal to halt and reverse fibrogenic progression is to selectively turn ‘OFF’ GIV’s GEF function (using small molecule inhibitors). C) Schematic summarizing our and others’ work (78, 86, 91) on how GIV regulates several steps of the insulin signaling cascade, e.g., receptor autophosphorylation, the recruitment and activation of IRS1, activation of the PI3K-Akt signaling pathway, exocytosis of the glucose transporter, GLUT4 and glucose uptake into skeletal muscles, all of which together constitute the metabolic insulin response. While an intact and efficient metabolic insulin response is required for physiology (i.e., insulin sensitivity), aberrant signals in response to circulating free fatty acids (among other causes) dampen such metabolic response and contribute to insulin resistance, the hallmark of type II diabetes. The metabolic insulin response is driven more efficiently and consequently, the insulin sensitivity is highest when GIV expression is elevated (78) and its GEF function is ‘ON’ (86). Fatty acids trigger insulin resistant states by phosphoinhibiting GIV’s GEF function via the DAG→PKCθ pathway, whereas insulin sensitizers like Pioglitazone act by potentiating tyrosine phosphorylation of GIV and by reversing phosphoinhibition and restoring GIV’s GEF function. Thus, the pharmacologic goal to reverse insulin resistance and reinstate insulin sensitivity is to selectively turn ‘ON’ GIV’s GEF function (by using positive allosteric modulators of GIV’s GEF function or by using specific inhibitors of PKCθ). D) Schematic summary of plausible therapeutic strategies to regulate GIV-dependent signaling. Among the currently available options are inhibitors (shown in yellow boxes) of the various upstream factors which coordinately potentiate GIV-dependent signaling, e.g., receptor and non-receptor tyrosine kinases that trigger tyrosine phosphorylation of GIV, or STAT3 which activates GIV transcription in diseased states, or the Ser/Thr kinase CDK5 which phosphoactivates GIV’s GEF function. Although selective inhibition of GIV’s GEF function using small molecules still remains unrealized, the therapeutic relevance of GIV depletion (by si/shRNA) or targeted disruption of its GEF function using cell permeable peptides has been experimentally validated (blue boxes).