Abstract
Immunotoxins are targeted anti-cancer therapeutics that kill cancer cells using a cytotoxic bacterial toxin payload. Their development for use in solid tumor malignancies was delayed due to issues with their immunogenicity and limited therapeutic window. However, new research has rejuvenated the field. Co-administration with a lymphocyte depleting regimen of pentostatin and cyclophosphamide can delay anti-drug antibody formation, increasing the number of treatment cycles that patients can receive and resulting in durable responses in heavily pre-treated patients. In addition, a new generation of immunotoxin molecules with reduced immunogenicity and non-specific toxicity has been developed through protein engineering techniques and one has recently entered the clinic. In pre-clinical studies in mouse models these new agents are effective against many tumor types as single agents, and also produce synergistic anti-tumor responses in combination with chemotherapy. These new immunotoxins have renewed excitement in the field and may prove a promising addition to the targeted therapy repertoire.
Keywords: Mesothelioma, Mesothelin, Cancer treatment, Pseudomanas exotoxin A, Immunosuppression, De-immunization
Antibody based therapies have revolutionized cancer treatment by allowing very specific targeting of cancer antigens. Conjugating cytolytic agents to antibodies allows for specific delivery of these agents to tumors. Antibody-drug conjugates (ADCs) carry a chemotherapy drug payload to cancer cells and have demonstrated success in breast cancer and Hodgkins lymphoma (1, 2). Immunotoxins are very potent molecules that consist of an antibody or antibody fragment linked to a bacterial or plant toxin rather than a traditional chemotherapeutic (3). Once the immunotoxin binds to the target tumor antigen it is internalized, undergoes processing and ultimately inhibits protein synthesis leading to cell death. Although immunotoxins targeting CD22 have produced complete remissions in refractory hairy cell leukemia and acute lymphoblastic leukemia in children (4, 5), they have been much less effective in targeting solid tumors. One reason for this lack of activity is the development of neutralizing antibodies to the toxin, limiting retreatment of patients. Another is the development of dose limiting capillary leak syndrome. Since the toxin is a foreign protein it elicits a strong host immune response that limits treatment to one cycle of three doses in most patients with solid tumors. Previous studies using immunosuppressive drugs such as steroids, cyclosporine, single agent cyclophosphamide or rituximab did not prevent development of antibodies.
Despite success in treating some hematologic malignancies where the immune system is suppressed, immunogenicity has been a large barrier to the development of clinically useful immunotoxins for solid tumors. Consequently progress in developing these molecules for solid tumors has been slow. However, our group has recently reported major cancer regressions in patients with mesothelioma treated with an immunotoxin and immune suppression. In addition, we have employed protein engineering to generate recombinant immunotoxins that are inherently less immunogenic. These developments could have broad implications for rejuvenating the field of immunotoxin cancer therapy.
Our laboratory uses protein engineering to produce recombinant immunotoxins. These are chimeric proteins that consist of the Fv fragment of an antibody reacting with a cancer cell fused to a truncated form of Pseudomonas exotoxin A (PE). Native PE has three functional domains: Domain I, which enables PE to bind to the surface of most cells, Domain II, which enables the toxin to be processed by furin separating the Fv from the toxin, and Domain III, which catalyzes the inactivation of elongation factor 2 leading to inhibition of protein synthesis and cell death (3). Using recombinant DNA technology, we removed Domain I (and additional unnecessary sequences) to produce a truncated PE toxin (PE38) that by itself cannot kill cells. To target the toxin to cancer cells, we replaced Domain I with an Fv selected to react with a cancer cell antigen (6, 7).
Immunotoxins are highly targeted therapies like antibody-drug conjugates, however, using a toxin payload rather than a chemotherapy payload to kill cancer cells results in some unique properties (see Table 1). Most importantly, immunotoxins are ideal to give in combination with standard chemotherapy. Immunotoxins kill cells by irreversibly modifying and inactivating elongation factor-2 to halt cellular protein synthesis, a non-overlapping mechanism of action from any standard chemotherapy agent. In addition, the main toxicity of immunotoxins, vascular leak syndrome, does not overlap with typical side effects from standard chemotherapies. For this reason, these two classes of drugs can be co-administered in the clinical setting with unmodified dosages of both the chemotherapy and the immunotoxin (8). In pre-clinical models this results in synergistic anti-tumor efficacy (9–11). Another special property of the toxin payload is that it can kill both actively dividing and quiescent cells, since all cells require protein synthesis for survival. This makes it especially critical that the tumor antigens targeted by immunotoxin therapeutics have very low or absent expression on normal cells, or expression confined to non-essential tissues, because the toxin also exposes normal cells that express the target antigen to the same cytotoxicity. For example, targeting Her2 with an immunotoxin resulted in severe liver toxicity due to minimal expression of Her2 on normal hepatocytes, while the ADC ado-trastuzumab maytansine is very well tolerated (12). However, this property also makes immunotoxins potentially useful for targeting less replicatively active cancer cell types and tumors that are inherently resistant to chemotherapy, such as hepatocellular carcinoma (13).
Table 1.
Comparison of ADC and Recombinant Immunotoxin Therapeutics
| ADC | Immunotoxin | |
|---|---|---|
| Targeting moiety | Full monoclonal antibody | Antibody fragment |
| Payload | Chemotherapy drug | Bacterial or plant toxin |
| Mechanism of Action | Most commonly anti-tubulin agents | Protein synthesis inhibition |
| Toxicity | Target specific, and most commonly peripheral neuropathy, myelosuppression | Target specific and vascular leak syndrome |
| Approved drugs in class | Trastuzumab emtansine (for HER-2 positive breast cancer); Brentuximab vedotin (for Hodgkins lymphoma and systemic anaplastic large cell lymphoma) | Denileukin diftitox (for cutaneous T cell lymphoma) |
| Advantages | Demonstrated efficacy in refractory setting Better tolerated than most standard chemotherapy Can kill tumor cells that don’t express target through bystander effect |
Can kill quiescent and rapidly dividing cells as a single agent Ideal for combination with standard chemotherapy due to non-overlapping mechanism of action and toxicity profile No off-target toxicity from bystander effect |
| Disadvantages | Overlapping mechanism of action to standard chemotherapy Cumulative peripheral neuropathy |
Immunogenicity Target selection more restrictive since payload is effective against many quiescent normal cells |
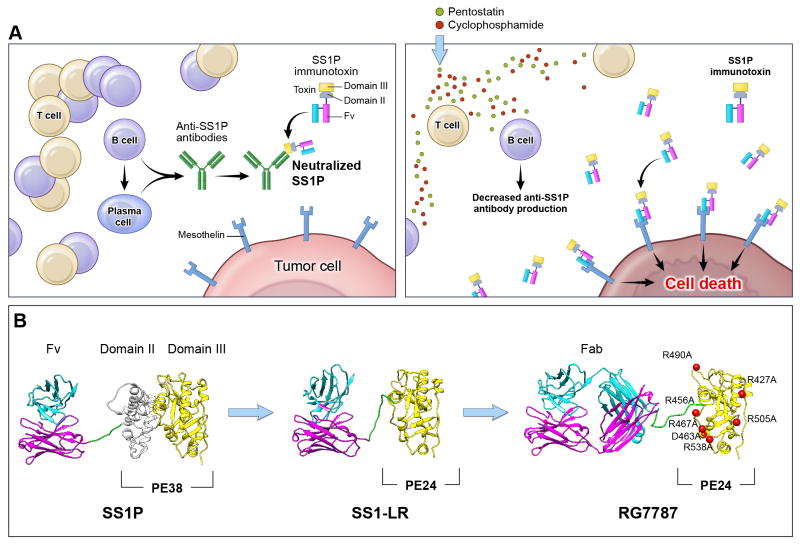
Our current clinical studies are focused on treating mesothelioma with the anti-mesothelin immunotoxin SS1P. Mesothelin is a lineage-restricted cell surface protein present on normal mesothelial cells, but highly expressed in many human malignancies including mesothelioma, and cancers of the pancreas, lung, stomach, ovary and bile duct (14). SS1P consists of an anti-mesothelin Fv linked to PE38. In a Phase I clinical trial SS1P had limited clinical activity by itself. One reason for low activity was that about 90% of patients developed antibodies to SS1P after just three doses and could not be retreated. Since T and B cells play an important role in antibody production, we evaluated an immunosuppressive regimen that targets both T and B cells to decrease the host immune response against immunotoxins. Using immunocompetent mice we showed that pre-treatment with pentostatin (that kills T cells) and cyclophosphamide (that kills B cells) abolished anti-SS1P antibody formation (15). To evaluate if such a strategy could work in humans, we carried out a pilot study in patients with advanced treatment refractory mesothelioma using pentostatin, cyclophosphamide and SS1P together. As predicted, this regimen significantly decreased formation of anti-SS1P antibodies allowing more cycles of SS1P to be given (16). Surprisingly, this regimen also induced durable and major tumor regressions in three of the ten evaluable patients with chemo-refractory disease. All three patients who had a response were alive more than two years from start of therapy. This strategy of combining immunotoxin therapy with pentostatin plus cyclophosphamide could be useful for immunotoxins targeting different tumor antigens and opens up the field of immunotoxin therapy for solid tumors (Figure 1A).
Figure 1. Avoiding the anti-immunotoxin antibody host response.
Decreasing the human immune response to immunotoxins can be accomplished either by manipulating the host immune system or by protein engineering of the toxin moiety. Panel A: After treatment with SS1P alone, patients develop anti-SS1P antibodies made by B and plasma cells. When patients are re-treated with SS1P these pre-formed antibodies bind to SS1P and prevent it from reaching the tumor (left). Pre-treatment of patients with pentostatin and cyclophosphamide prior to SS1P administration depletes T and B cells and decreases the production of anti-SS1P antibodies (right). This prevents host neutralization of SS1P with repetitive dosing and allows more SS1P to reach tumor cells. Panel B: Another approach is to modify the PE toxin to remove immunogenic epitopes so that the toxin is inherently less immunogenic. Structural models of SS1P and its de-immunized variants are shown. The targeting domain consists of VL (cyan) and VH (magenta). The linker between the targeting domain and PE contains the furin cleavage site (green), which is required for toxin cytotoxic activity. The furin cleavage site is part of PE Domain II. The remainder of Domain II (gray) is unnecessary for cytotoxicity and has been deleted in the PE24-based toxins, SS1-LR and RG7787. Domain III (yellow) is the catalytic domain of PE. In RG7787, alanine point mutations were introduced at seven bulky hydrophilic residues (red) to silence human B cell epitopes within this domain. Deletion of Domain II reduces the size of the molecule into the range where it can be easily filtered by the kidneys, reducing serum half-life. RG7787 (aka Ro6927005) contains a larger humanized Fab for targeting which raises its molecular weight above this threshold. This molecule has significant anti-tumor activity in pre-clinical models and has now begun Phase I clinical testing.
Since the toxin used to make the immunotoxin is a foreign protein, identifying and mutating its immunogenic epitopes should produce therapeutic molecules that are less immunogenic. We have identified the majority of the B and T cell epitopes in PE38 and used protein engineering to remove or silence these epitopes (Figure 1B). We found that most of Domain II can be deleted from PE as long as 11 amino acids containing the furin-processing site are retained. This deletion removes the B and T cell epitopes in Domain II and unexpectedly the mutant immunotoxin (SS1-LR) is usually more active than SS1P in killing cancer cells. It is also about 8-fold less toxic to mice and has a greatly diminished ability to produce capillary leak syndrome in rats (17). Apparently Domain II is responsible for these undesirable properties. To silence B cell epitopes in Domain III we mutated seven bulky hydrophilic residues to alanine (18). Since molecules without Domain II are small, about 43-kDa, they are rapidly filtered and removed from the circulation by the kidneys. To increase latency in the blood, we have collaborated with Roche Diagnostics GmbH to produce RG7787 (Ro6927005). This protein has a molecular weight of 72-kDa and contains a humanized anti-mesothelin Fab fused to the mutated toxin (Figure 1B, Table 2). RG7787 is very active against a variety of mesothelin expressing cell lines and is more active than SS1P against cells directly isolated from mesothelioma patients (R.H. unpublished data). In vivo, it has a much larger therapeutic window than SS1P due to the decreased toxicity and therefore improved in vivo efficacy even in xenograft models utilizing cell lines with nearly identical in vitro sensitivity to SS1P and RG7787. Also, when RG7787 was combined with paclitaxel, it produced complete or near-complete remissions in a pancreatic cancer model in mice (10, 11). RG7787 is currently undergoing Phase I clinical testing in patients with mesothelin-positive malignancies including mesothelioma, ovarian, pancreatic, gastric and triple negative breast cancers (NCT02317419).
Table 2.
Comparison of SS1P and the Redesigned Anti-Mesothelin Immunotoxin RG7787
| SS1P | RG7787 | |
|---|---|---|
| Targeting moeity | Anti-MSLN dsFv | Humanized anti-MSLN Fab |
| PE payload | PE containing Domains II and III (PE38) | PE with most of Domain II deleted and Domain III bearing 7 point mutations to remove B cell epitopes (PE24) |
| Payload size | 38-kDa | 24-kDa |
| Full molecule size | 62-kDa | 72-kDa |
| Activity in vitro | Picomolar range for many tumor cell lines | Picomolar range for many tumor cell lines |
| Mouse MTD | 0.4 mg/kg I.V. every other day x3* | 3.75 mg/kg I.V. every other day x3* |
| Efficacy in mouse tumor models | Shrinks A431 epidermoid cancer cells expressing transfected mesothelin and complete regressions with chemotherapy (9). Ineffective against KLM1 pancreatic.* |
Decreases tumor volume of mesothelin-positive breast (HCC70), gastric (MKN28), and large lung tumors (H596). Cytostatic in KLM1 pancreatic as single agent, but complete regressions with taxane (10, 11, 19) |
| Immunogenicity | 90% of patients make neutralizing anti-drug antibodies after 1 cycle (20, 21) | Limited reactivity to anti-drug antibodies from sera of patients previously treated with SS1P (18) |
| Current clinical testing | Phase II in combination studies with pentostatin and cyclophosphamide (NCT01362790) | Phase I for mesothelin-positive tumors (NCT02317419) |
unpublished
MSLN, mesothelin; MTD, maximum tolerated dose
In summary, we have developed new immunotoxins that are designed to be less immunogenic and more active. Secondly, clinical trial results show that the human anti-drug immune response to an immunotoxin can be diminished to allow for repeat dosing. Given on this schedule immunotoxin treatment can produce dramatic tumor responses in some patients. These efforts herald an exciting development for the field. Larger clinical trials over the next few years will be needed to realize the original promise of immunotoxin therapy.
Translational relevance.
Most anti-cancer monoclonal antibodies have little cytotoxic activity but conjugation to tubulin inhibiting drugs to produce antibody-drug conjugates has proven beneficial in some tumor types. Another approach to make anti-cancer antibodies therapeutically useful is linking them to potent bacterial toxins that kill tumor cells by inhibition of protein synthesis. These immunotoxins have shown single agent activity in some hematological cancers but limited efficacy in solid tumors. The principal reason for their lack of activity is that their immunogenic nature limits retreatment of patients. However, newer immunotoxins generated by protein engineering are inherently less immunogenic and also more active. This increases the likelihood that patients with solid tumors can receive multiple cycles of therapy, resulting in increased anti-tumor activity. RG7787 is a de-immunized immunotoxin targeting mesothelin has just entered clinical trials for treatment of mesothelin expressing cancers.
Acknowledgments
Support
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest
I. Pastan is the inventor on immunotoxin patents that are all assigned to the NIH. Other authors declare no potential conflicts of interest.
Authors’ Contributions:
Conception and design: R.H., I.P.
Analysis and Interpretation of data: R.H., C.A., I.P.
Writing and review of manuscript: R.H., C.A., I.P.
References
- 1.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Eng J Med. 363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 3.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 4.Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN, et al. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: phase I study in pediatric acute lymphoblastic leukemia (ALL) Blood (ASH Annual Meeting Abstract) 2011;118:248. [Google Scholar]
- 5.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreitman RJ, Siegall CB, Chaudhary VK, FitzGerald DJ, Pastan I. Properties of chimeric toxins with two recognition domains: interleukin 6 and transforming growth factor alpha at different locations in Pseudomonas exotoxin. Bioconjug Chem. 1992;3:63–8. doi: 10.1021/bc00013a010. [DOI] [PubMed] [Google Scholar]
- 7.Batra JK, Kasprzyk PG, Bird RE, Pastan I, King CR. Recombinant anti-erbB2 immunotoxins containing Pseudomonas exotoxin. Proc Natl Acad Sci USA. 1992;89:5867–71. doi: 10.1073/pnas.89.13.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M, et al. Phase I study of the antimesothelin immuotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120:3311–9. doi: 10.1002/cncr.28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xiang L, Hassan R, Paik CH, Carrasquillo JA, Jang BS, et al. Synergistic antitumor activity of Taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 10.Hollevoet K, Mason-Osann E, Liu X, Imhof-Jung S, Niederfellner G, Pastan I. In vitro and in vivo activity of the low-immunogenic anti-mesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–9. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alewine C, Xiang L, Yamori T, Niederfellner G, Bosslet K, Pastan I. Efficacy of RG7787, a next generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–61. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai-Scherf LH, Villa J, Pearson D, Watson T, Liu E, Willingham MC, et al. Hepatotoxicity in cancer patients receiving erb-38, a recombinant immunotoxin that targets the erbB2 receptor. Clin Cancer Res. 1999;5:2311–5. [PubMed] [Google Scholar]
- 13.Gao W, Tang Z, Zhang YF, Feng M, Qian M, Dimitrov DS, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 2015;6:6536. doi: 10.1038/ncomms7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–28. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Mossoba ME, Onda M, Taylor J, Treadwell S, Sharon E, Hassan R, et al. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;11:3697–705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldon JE, Xiang L, Zhang J, Beers R, Walker DA, Onda M, et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Onda M, Lee B, Kreitman RJ, Hassan R, Xiang L, et al. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA. 2012;109:11782–7. doi: 10.1073/pnas.1209292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederfellner G, Bauss F, Imhof-Jung S, Hesse F, Kronenberg S, Staak R, et al. RG7787-a novel de-immunized PE based fusion protein fo therapy of mesothelin-positive solid tumors. Proceedings of the 105th Annual Meeting of the AACR; 2014. p. 4510. abstr. [Google Scholar]
- 20.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 21.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]