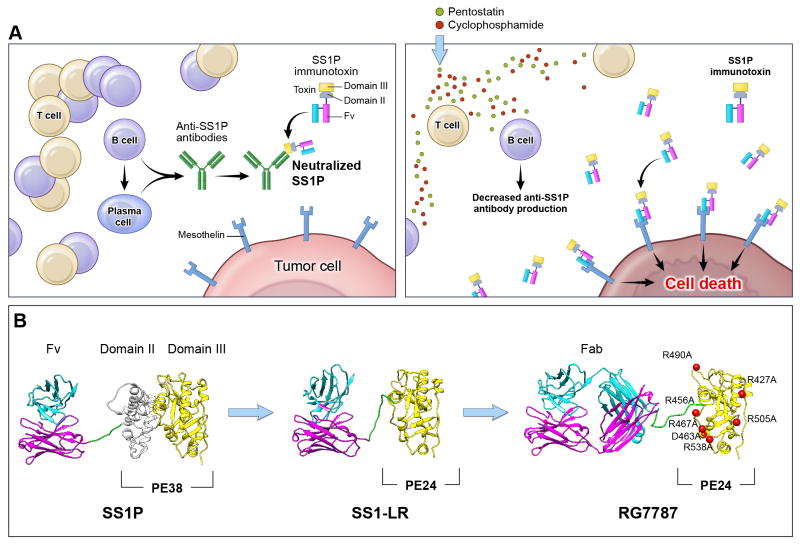
Figure 1. Avoiding the anti-immunotoxin antibody host response.
Decreasing the human immune response to immunotoxins can be accomplished either by manipulating the host immune system or by protein engineering of the toxin moiety. Panel A: After treatment with SS1P alone, patients develop anti-SS1P antibodies made by B and plasma cells. When patients are re-treated with SS1P these pre-formed antibodies bind to SS1P and prevent it from reaching the tumor (left). Pre-treatment of patients with pentostatin and cyclophosphamide prior to SS1P administration depletes T and B cells and decreases the production of anti-SS1P antibodies (right). This prevents host neutralization of SS1P with repetitive dosing and allows more SS1P to reach tumor cells. Panel B: Another approach is to modify the PE toxin to remove immunogenic epitopes so that the toxin is inherently less immunogenic. Structural models of SS1P and its de-immunized variants are shown. The targeting domain consists of VL (cyan) and VH (magenta). The linker between the targeting domain and PE contains the furin cleavage site (green), which is required for toxin cytotoxic activity. The furin cleavage site is part of PE Domain II. The remainder of Domain II (gray) is unnecessary for cytotoxicity and has been deleted in the PE24-based toxins, SS1-LR and RG7787. Domain III (yellow) is the catalytic domain of PE. In RG7787, alanine point mutations were introduced at seven bulky hydrophilic residues (red) to silence human B cell epitopes within this domain. Deletion of Domain II reduces the size of the molecule into the range where it can be easily filtered by the kidneys, reducing serum half-life. RG7787 (aka Ro6927005) contains a larger humanized Fab for targeting which raises its molecular weight above this threshold. This molecule has significant anti-tumor activity in pre-clinical models and has now begun Phase I clinical testing.