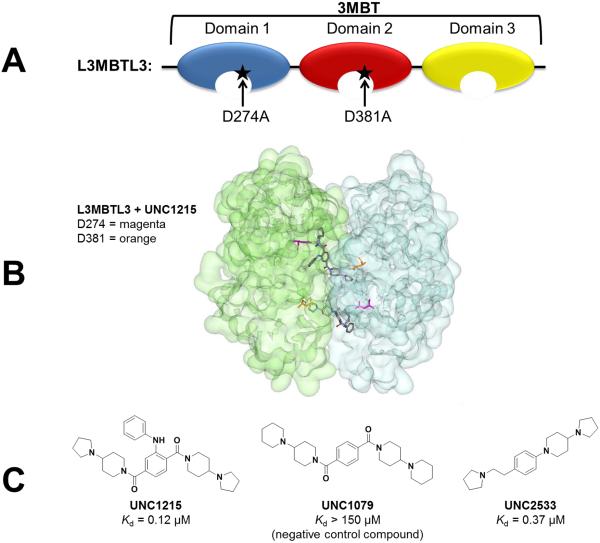
Figure 1.
L3MBTL3 and its small molecule ligands. A) The 3MBT domains of L3MBTL3 were used in biochemical assays to discover the small molecule probe UNC1215. Mutations to domain 1 (D274A) and domain 2 (D381A) decreased or abolished the binding of UNC1215 to L3MBTL3 respectively. B) The x-ray crystal structure of the 2:2 dimer of L3MBTL3 and UNC1215 shows a face to face arrangement wherein two copies of protein (one copy shown in green and one in blue) sandwich two ligand molecules (pdb 4FL6). Residue D274 is highlighted in magenta and residue D381 is highlighted in orange. C) Structures of small molecule inhibitors of L3MBTL3 and their affinities as measured by isothermal titration calorimetry. UNC1079 is a structurally similar but significantly less potent negative control compound.