Abstract
Lymphangiomas of the adrenal glands (ALs) are benign vascular lesions. Approximately, 53 cases have been reported in the literature. The current study reviews and analyzes the clinical and pathologic features of all reported ALs and additionally illustrates a typical case of adrenal lymphangioma (AL). In order to perform the review analysis, a search of the international literature for ALs in adults was conducted. Thirty-eight related articles were found. Clinical and pathological information were obtained for all the reported cases and a database was created. ALs were detected more frequently in women than men. The mean age of occurrence was 39.5 years, while their mean size was 8.86 cm. Fifty-nine percent of ALs were right-sided. Size and localization were responsible for the presenting symptoms, though 30.4 % were asymptomatic. Diagnosis was made postoperatively in all cases by histological results. ALs are rare and benign lesions. They usually present as an incidental finding after abdominal imaging. The diagnosis is made after the surgical removal by histological and immunohistochemical examinations.
Keywords: Lymphangioma, Adrenal tumor, Incidentaloma, Adrenal cyst
Introduction
Adrenal cysts are rare lesions. They can be classified as endothelial cysts (45 %), pseudocysts (39 %), epithelial cysts (9 %), and parasitic cysts (7 %) [1]. Endothelial cysts include hemangiomas, hamartomas, and lymphangiomas [1]. Adrenal lymphangiomas (ALs) are rare benign vascular lesions that usually remain asymptomatic throughout life [2]. They are usually unilateral, variable in size, with smooth borders, and pure cystic internal structure [2]. To date, only a few cases have been reported in the literature.
The aim of this study is to review the literature in order to determine clinical features, pathological findings, and management of these lesions. Additionally, we present a patient with a typical AL in the right adrenal gland.
Materials and Methods
An electronic search of PubMed/Medline database using the medical subjected headings (MeSH) was performed, using combinations of the following keywords/phrases: adrenal cystic tumors; adrenal tumor; incidentalomas; adrenal cyst; adrenal lymphangiomas. Original research papers, case reports, and review articles were included.
Searching the international literature since 1965, when Lynn [3] reported one of the first known AL, to 2014, 38 articles describing 53 cases of ALs in adults (>16 years) were found [1–39]. The majority of these articles were single case reports. It is noteworthy that 15 cases were reported in two articles, one by Ellis et al. [2] (nine cases) and the other by Chien et al. [34] (six cases). Berthet et al. [15] mentioned three cases; however, one of the described patients was previously reported by the same authors [40]. Constantino et al. [14, 41] reported the same patient twice, whereas Bisceglia et al. [37] described a case of lymphangioma like adenomatoid tumor and was excluded from our analysis. In five cases, we were unable to obtain the data and were also excluded from the analysis [4, 6, 9, 18, 20].
The review analysis included 31 articles which describe 48 AL cases. Table 1 shows in details all the reported ALs. Each article was carefully studied and a database was created. This database included 13 characteristics of ALs such as gender, age, presenting symptoms, tumor size and localization, laboratory findings, diagnostic imaging modalities, treatment, malignancy, pathological and immunohistochemical findings, and follow-up surveillance.
Table 1.
Adrenal lymphangiomas (ALs)
| Case | Author/year | Sex/age | Site | Size | Symptoms & signs | Hormone secreting | Imaging modalities | Treatment | Pathology/immunohistochemistry | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lynn [3]/1965 | M/50 | Left | 5.0 | Hypertension, headaches, dizziness, nausea, lassitude | No | Urography, angiography | Open adrenalectomy | CL | 24 |
| 2 | Boscaino [5]/1977 | F/21 | Right | NR | Urinary infection symptoms, Palpable mass | No | Urography, angiography | Open adrenalectomy | CL | NR |
| 3 | Georgi [7]/1982 | F/48 | Right | 12.0 | Pain in right upper abdomen, hypertension | No | U/S, angiography | Open adrenalectomy | CL | NR |
| 4 | Millon [8]/1982 | M/45 | NR | NR | Hypertension | NR | U/S, angiography | Open adrenalectomy | CL | NR |
| 5 | Pfister [10]/1987 | F/51 | Right | 5.0 | Lumbar pain | Iodocholesterol | Angiography | Open adrenalectomy | CL | NR |
| 6 | Gossot [11]/1987 | F/39 | Left | 10.0 | Fever, incidental adrenal mass | No | U/S, CT, angiography | Open adrenalectomy | CL | 3 |
| 7 | Sanromá [12]/1988 | F/20 | Right | 2.5 | Epigastric & upper right quadrant pain, nausea, vomiting | No | U/S, CT | Open adrenalectomy | CL | NR |
| 8 | Gleeson [13]/1988 | F/38 | Right | 7.0 | Right loin pain, weight loss | No | U/S, CT, urography, angiography | Open adrenalectomy | Multiple CL | NR |
| 9 | Costantino [14]/1993 | M/16 | Left | 10.0 | Epigastric pain | No | U/S, CT, urography | Open adrenalectomy | CL | NR |
| 10 | Berthet [15]/1993 | M/57 | Right | 10.0 | Hypertension | No | U/S, CT, urography | Open resection | CL | NR |
| 11 | Berthet [15]/1993 | F/32 | Right | 30.0 | Right hypochondrium mass | No | U/S, CT, angiography | Open adrenalectomy | CL | NR |
| 12 | Berthet [15]/1993 | F/33 | Right | 13.0 | Incidental adrenal mass | No | U/S, CT | Open resection | Cystic haemolymphangioma | NR |
| 13 | Camara [16]/1994 | F/25 | Right | 14.0 | Right upper quadrant pain, nausea | No | U/S | Open adrenalectomy | CL | NR |
| 14 | Iderne [17]/1995 | F/52 | Right | 12.0 | Right upper quadrant pain, nausea, vomiting | Aldosterone | U/S, CT, urography | Open partial adrenalectomy | CL | NR |
| 15 | Alapont Pérez [19]/1996 | F/34 | NR | NR | Lower urinary tract infection, continuous and aggravating pain in left lumbar area | No | U/S, CT, urography | Open adrenalectomy | CL | NR |
| 16 | Mortelé [21]/1996 | M/58 | Bilateral | R:21, L:4 | Diffuse abdominal pain, nausea, vomiting (syndrome Gorlin-Goltz) | No | U/S, CT, MRI | Open resection | CL | NR |
| 17 | Hoeffel [22]/1999 | F/22 | Bilateral | R:7.8, L:8.7 | Left upper abdominal pain, palpated mass | No | U/S, CT, MRI, urography | Open resection | CLs | NR |
| 18 | Longo [23]/2000 | F/30 | Right | 4.0 | Right flank discomfort | No | U/S, CT, MRI, urography | Open adrenalectomy | CL | 24 |
| 19 | Trojan [24]/2000 | M/40 | Right | 5.7 | Partial small-bowel obstruction | No | U/S, CT | Open resection | CL | NR |
| 20 | Yokota [25]/2000 | F/53 | Left | NR | Residual urine feeling | Catecholamines and cortisol | U/S, CT, MRI, urography, angiography | Open adrenalectomy | CL | NR |
| 21 | Touiti [26]/2003 | F/38 | Right | 9.4 | Chronic right lumbar pain | Aldosterone | U/S, CT, MRI, urography | Open subtotal adrenalectomy | CL | 3 |
| 22 | Satou [27]/2003 | M/46 | Left | 2.0 | Palpitation and siderosis | No | U/S, CT, MRI | Laparoscopic adrenalectomy | Multiple CL/LCA, CD20, factor VIII and CD34 IN endothelium | 24 |
| 23 | Luncă [28]/2004 | F/47 | Left | 12.0 | Incidental adrenal mass | No | U/S | Open resection | CL | NR |
| 24 | Robledo-Ogazón [29]/2004 | F/21 | Right | 7.0 | Hypertension, frontal headache, nausea, anxiety, irritability, depression | No | U/S, CT | Open adrenalectomy | Multiple CL | NR |
| 25 | Garcia [30]/2004 | F/22 | Left | 3.5 | Right lower quadrant abdominal pain | NR | U/S, CT, MRI | Open partial adrenalectomy | CL/CD 31 | NR |
| 26 | Ates [1]/2005 | F/26 | Right | 9.0 | Weakness, put weight, lumbago | No | U/S, CT, MRI | Open adrenalectomy | Multiple CL/CD31, CD34, smooth muscle actin | NR |
| 27 | Nouira [31]/2007 | F/30 | Left | NR | Incidental adrenal mass | NR | U/S, CT | Laparoscopic excision | CL | NR |
| 28 | Bettaïeb [32]/2007 | F/22 | Left | 35 | Left flank pain, nausea, vomiting, constipation | No | U/S, CT | Open subtotal adrenalectomy | CL/CD34, factor VIII | NR |
| 29 | Pereira [33]/2007 | F/41 | Left | 3.0 | Hypertension | Catecholamines | U/S, CT | Open resection | Multiple CL/CD31, CD34 | NR |
| 30 | Chien [34]/2008 | F/59 | Right | 6.0 | Incidental adrenal mass | Catecholamines | U/S, CT | Open adrenalectomy | CL/D2-40, CD34 | 16 |
| 31 | Chien [34]/2008 | F/43 | Right | 5.0 | Hypertension, dizziness | No | U/S, CT | Open adrenalectomy | CL/D2-40, CD34 | 26 |
| 32 | Chien [34]/2008 | M/50 | Left | 15.0 | Left flank soreness | No | U/S, CT | Open adrenalectomy | CL/D2-40, CD34 | 21 |
| 33 | Chien [34]/2008 | F/53 | Right | 7.0 | Flank pain | No | U/S, CT | Open adrenalectomy | CL/D2-40, CD-34 | 191 |
| 34 | Chien [34]/2008 | F/31 | Right | 18.0 | Painless abdominal mass | No | U/S, CT | Open adrenalectomy | CL/D2-40, CD-34 | 1 |
| 35 | Chien [34]/2008 | M/58 | Left | 5.7 | Incidental adrenal mass | No | U/S, CT | Open adrenalectomy | CL/D2-40, CD34 | 23 |
| 36 | Ait Ali [35]/2009 | F/33 | Right | 6.5 | Right flank pain, inferior edemas | NR | U/S, CT, MRI | Open resection | CL | NR |
| 37 | Cutaja [36]/2009 | F/60 | Left | 4.0 | Recurrent abdominal pain | NR | U/S, CT, MRI | Open adrenalectomy | CL | NR |
| 38 | Ellis [2]/2010 | F/51 | Right | 3.2 | Flank pain, hypertension | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40 | NR |
| 39 | Ellis [2]/2010 | M/38 | Right | 2.0 | Morbid obesity, hypertension | NR | U/S, CT, MRI | Partial adrenalectomy | CL/D2-40, CD31 | NR |
| 40 | Ellis [2]/2010 | F/28 | Left | 13.5 | Back pain, increasing adrenal mass | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40, CD31, VIMENTIN | NR |
| 41 | Ellis [2]/2010 | M/46 | Right | 4.0 | Urethral bleeding | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40, CD34 | NR |
| 42 | Ellis [2]/2010 | F/32 | Left | 6.5 | Abdominal pain, early satiety | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40 | NR |
| 43 | Ellis [2]/2010 | M/46 | Right | 4.0 | Incidental adrenal mass | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40, CD31 | NR |
| 44 | Ellis [2]/2010 | F/42 | Right | 6.5 | Increasing back pain, increasing size of adrenal mass | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40 | NR |
| 45 | Ellis [2]/2010 | F/39 | Right | NR | Florid endometriosis, incidental adrenal mass | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40, CD31, CD34, factor VIII | NR |
| 46 | Ellis [2]/2010 | F/56 | Left | 2.5 | Chest pain | NR | U/S, CT, MRI | Open adrenalectomy | CL/D2-40 | NR |
| 47 | Sallami [38]/2013 | F/29 | Right | 6.0 | Lumbar pain | No | Pyelography, U/S, CT | Open adrenalectomy | CL/CD31, CD34 | 17 |
| 48 | Liu [39]/2013 | F/45 | Left | 3.0 | Incidental adrenal mass | No | U/S, CT | Retroperitoneoscopic adrenalectomy | CL/CD31, CD34 | NR |
M male, F female, NR not reported, CL cystic lymphangioma, U/S ultrasonography, CT computed tomography, MRI magnetic resonance imaging
Results
Table 2 summarizes all ALs features. Thirty-six female and 12 male patients were reported (F/M: 3/1), and all patients were adults (>16 years) at the time of diagnosis. The most common symptoms were pain (47.9 %) and hypertension (14.6 %). Pain was localized in the back, flanks, right upper quadrant, or was generalized as abdominal pain. Hypertension seems to be unrelated with ALs. It is remarkable that 15 ALs were diagnosed incidentally, as patients were presented with unrelated or no complaints. Laboratory findings in six ALs cases showed hormones over secretion [10, 17, 25, 26, 33, 34]. The detected hormones were catecholamines, aldosterone, cortisol, and iodocholesterol. The majority of ALs (58.7 %) were right-sided. Mortele et al. [21] and Hoeffel et al. [22] reported two cases of bilateral ALs. The mean size of ALs was 8.7 cm (maximum diameter was used to determine the size of the cyst). Imaging modalities have misdiagnosed all the reported ALs. To date, ALs have been characterized mostly as incidentalomas (56.52 %) and rarely as adrenal adenomas [2, 26, 36], pheochromocytomas [2, 29, 33], or adrenal cysts [2, 5, 7]. Moreover, five ALs have been characterized as non-adrenal lesions [2, 14, 15, 17, 34]. Accurate diagnosis for all ALs was made postoperatively by pathology reports. Surgical excision was considered as the appropriate treatment in all reported ALs. Open transabdominal adrenalectomy was the procedure of choice. Satou et al. [27] and Nouira et al. [31] performed laparoscopic adrenalectomy, while Liu et al. [39] has performed recently a retroperitoneoscopic procedure.
Table 2.
Characteristics of adrenal lymphangiomas (ALs)
| N of patients | Percentage (%) | Mean | Range | ||
|---|---|---|---|---|---|
| Gender | Male | 12 | 25.0 | ||
| Female | 36 | 75.0 | |||
| Age (years) | 39.5 | 16–60 | |||
| Symptoms and signs | Pain | 23 | 47.9 | ||
| Hypertension | 7 | 14.6 | |||
| Palpable mass | 2 | 4.1 | |||
| Partial small-bowel obstruction | 1 | 2.1 | |||
| Unrelated or no complaints | 15 | 31.3 | |||
| Tumor size (diameter in cm) | 8.86 | 2.0–35.0 | |||
| Location | Right | 27 | 58.7 | ||
| Left | 17 | 36.9 | |||
| Bilateral | 2 | 4.4 | |||
| Type of cyst | Unilocular | 25 | 53.2 | ||
| Multilocular | 22 | 46.8 | |||
| Treatment | Open resection | 8 | 16.6 | ||
| Open partial adrenalectomy | 5 | 10.4 | |||
| Open adrenalectomy | 32 | 66.7 | |||
| Laparoscopic excision | 1 | 2.1 | |||
| Laparoscopic adrenalectomy | 1 | 2.1 | |||
| Retroperitoneoscopic adrenalectomy | 1 | 2.1 | |||
| Malignancy | No | 46 | 95.9 | ||
| Possible | 2 | 4.1 | |||
| Follow up (months) | 32.4 | 1–191 | |||
Macroscopically, the lesions were mostly unilocal and described as cystic, while two lesions were characterized as cystic haemolymphangiomas [11, 15]. Potency of malignant transformation was described in two cases [2, 27] with atypical lymphocytes and marked degenerative changes. Data concerning the immunohistochemistry were available in 20 patients. Immunostains included CD31, CD34, factor VIII, and D2-40. Regarding the two ALs associated with lymphocyte proliferation, the one was found positive to CD20 and LCA stains [27], while the other had positive reactivity for CD20, CD79a, CD43, and B cell lymphoma-2 stains [2]. Overall, for both cases, the cells were regarded as atypical, but the lymphoid process could not be further characterized because of the diffused degenerative cytologic changes [2, 27]. In all patients having available follow-up surveillance data, the postoperative period was uneventful. Lymphoproliferative process was not detected in the two ALs associated with atypical cells [2, 27].
Case Presentation (Illustrative Case of AL)
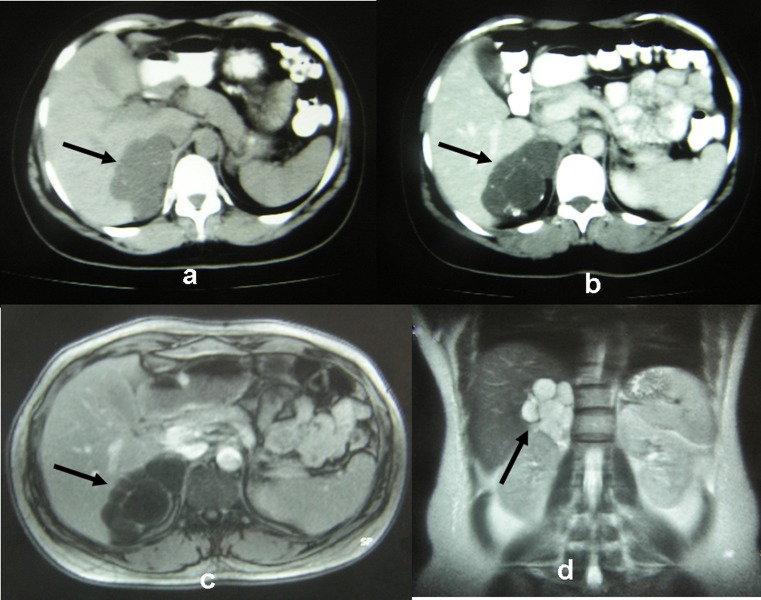
We report a 39-year-old woman with a previous history of head melanoma excision. Staging abdominal computed tomography (CT) revealed a lesion of 9.0 × 6.5 × 4.0 cm located on the right adrenal gland. It appeared to be of water density with loculi lacking of enhancement (Fig. 1a, b). The multi-cystic lesion seemed to displace the right liver lobe as shown in magnetic resonance imaging (MRI) (Fig. 1c, d). Serum and urine analyses were not indicative of a functioning adrenal tumor. The patient’s medical history of melanoma and the size of the lesion (>6 cm) were considered as risk factors for malignancy and therefore the surgical removal of right adrenal gland was considered appropriate. The patient was scheduled for open adrenalectomy via transabdominal approach. The duration of operation was 85 min, and the cystic lesion was excised en-block with the right adrenal gland, while macroscopically no infiltration of the surrounding tissues was observed (Fig. 2). The postoperative period was uneventful, and the patient was discharged on the fifth postoperative day.
Fig. 1.
a, b CT images are showing a lesion of 9.0 × 6.5 × 4.0 cm located on the right adrenal gland. c, d MRI images are showing a multi-cystic lesion on the right adrenal gland. All arrows are indicating the cystic lesion
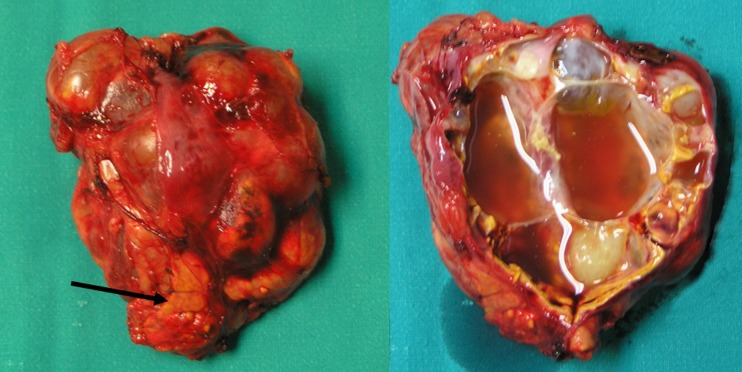
Fig. 2.
Surgical specimen, cystic tumor of the right adrenal gland. Arrow is indicating the adrenal tissue
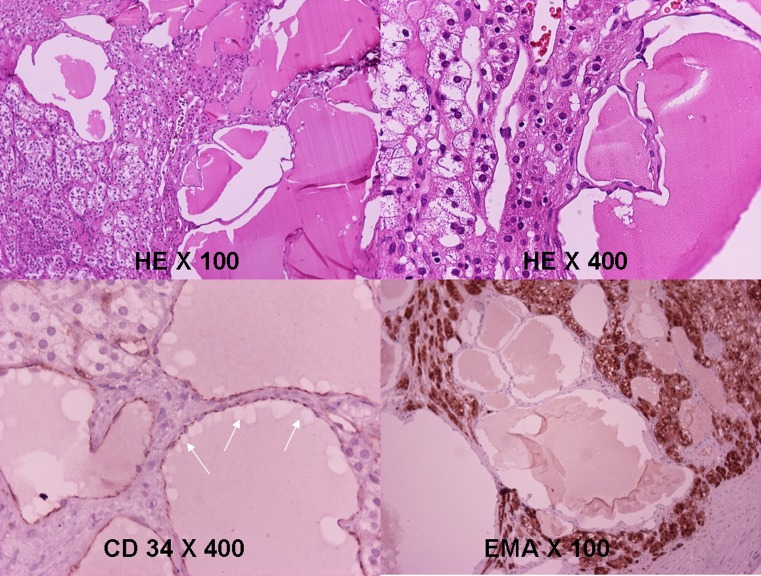
Histological examination revealed a multi-cystic lesion with flat endothelial cells adjacent to the normal-appearing adrenal cortex (Fig. 3). Immunohistochemical staining results showed that the cells were positive for CD31, CD34, and D2-40 and negative for cytokeratines (Fig. 3). Based on histopathological results, the diagnosis of cystic AL was made.
Fig. 3.
The mass consists of dilated eosinophilic material-containing spaces. A layer of cells cover the inner surface (HE ×100, HE ×400). Cells stain positive for CD 34 and negative for EMA. HE hematoxylin–eosin, EMA epithelial membrane antigen
The patient’s follow up included CT on the sixth postoperative month and afterwards annually. Two years after surgery, the patient remains free of symptoms.
Discussion
Lymphangiomas are benign malformations of vessels, most frequently discovered in childhood [1]. The cause of lymphangiomas has not been established. They are generally regarded as congenital malformations in which obstruction or agenesis of lymphatic tissue results in lymphangiectasia due to lack of normal communication of the lymphatic system [42]. Other authors believe that these tumors arise from continuous growth of ectopic or malformed lymphatic tissue, or represent a hyperplastic reaction to inflammation, or a lymphatic hamartoma [22]. They are usually located in the neck, axillary region, and mediastinum (95 %) [43], whereas the remaining 5 % appear in other parts of the body such as lungs, pleura, pericardium, gastrointestinal system, pancreas, liver, gallbladder, mesentery kidney, and adrenal glands [44]. ALs are uncommon lesions, and their pathogenesis is the same as in the other lymphangiomas [1].
Clinical presentation is not typical for ALs, whereas one third of the cases were incidentally detected. They are usually presented in young women, but there is no formal explanation for that female predominance. Laboratory findings are also nonspecific and usually not helpful as a diagnostic tool. In six cases, hormone hypersecretion was reported [10, 17, 25, 26, 33, 34]. Since ALs are not hormone-productive lesions, it is remarkable that hypersecretion findings were presented in these cases. Nevertheless, pathology reports were not indicative of functional adrenal tumors.
The widespread use of diagnostic images modalities such as CT and MRI has facilitated the diagnosis of adrenal neoplasms including ALs. However, accurate diagnosis was not made preoperatively and they were characterized as incindentalomas. Ultrasonography, CT, and MRI are the modalities used in the evaluation of adrenal cystic lesions [45]. Ultrasound often demonstrates a well-marginated lesion. If calcification or internal debris is present, ultrasound may show more complicated appearance with acoustic shadowing and internal echoes [23]. On CT, adrenal cystic lesions are characterized by lack of enhancement with intravenous contrast. The cystic fluid measures water density or higher if hemorrhagic or protein component is presented [46]. On MRI, they are low in signal intensity on T1-weighted images and high on T2-weighted images [23, 47]. Pheochromocytoma, neuroblastoma, adenomatoid tumor, schwannoma, rare cases of metastasis in adrenal glands (i.e., breast cancer), and rare cases of primary adrenal gland malignant lymphoma can present as cystic lesions and should be included into the differential diagnosis of ALs [45]. Cyst’s internal texture, wall thickness, calcification pattern, absence of a true contrast enhancement, positive clinical/laboratory findings, and patient history should all be considered in making the diagnosis [44].
Clinical management of ALs can be aided by the imaging findings. Lesion diameter is the main indication for AL removal. However, there is no consensus in the literature regarding the optimal size of the tumor in need of surgical intervention [48]. Small asymptomatic ALs with clear fluid can simply be observed, whereas large symptomatic lesions should be excised [32]. Some authors recommend aspiration of the contents of adrenal cysts both for diagnosis and management instead of surgical excision, if the suspicion of malignancy is low, or the lesion is non-functional and asymptomatic [1]. This method, though, is characterized by the high re-accumulation of the cyst fluid and probably the dispersion of malignant cells in the peritoneal cavity, while its ability to determine the histology of cyst is limited. However, it may be the best and only option in a patient who is at high surgical risk [23].
Surgery should be the appropriate treatment for large ALs. For many years, open transabdominal adrenalectomy had been applied for the surgical removal of these neoplasms. This procedure requires a relatively large incision due to the deep retroperitoneal location of the adrenal glands [48]. Laparoscopic excision, though, seems to be particularly suitable as most adrenal tumors are small and pathologically benign [49]. Laparoscopic surgery is feasible for all benign adrenal tumors even in those with large size (>8 cm) [50]. The benefits of laparoscopic adrenalectomy are reduced blood loss, lower requirements for analgesia, shorter hospital stay, and quicker recovery. In view of this, it is suggested that minimally invasive adrenalectomy might lead to a better clinical outcome, as compared to the open surgery [46, 48].
At pathological studies, ALs have a recognizable endothelial lining. Multiloculated cystic and endothelial lined cavities with lymphocyte aggregation are focally observed on the cyst’s wall [27]. The content of the cyst is mainly proteinaceous [27]. Immunohistochemical studies are also used to establish the diagnosis. The endothelial lining stains positive for factor VIII-related antigen, CD31 and CD34, while it demonstrates a lack of staining for cytokeratin. These findings confirm the lymphatic rather than the mesothelial nature of the lining [1]. ALs are also stained positive by the relatively novel D2-40. This is a monoclonal antibody directed against the human podoplanin, a transmembrane mucoprotein expressed by lymphatic endothelial cells among others [51]. Unlike other vascular markers, such as CD31 and CD34, that label both blood vessel and lymphatic endothelium, D2-40 immunoreactivity is restricted to lymphatic endothelium, thus, it is as a more specific marker of lymphatic lineage [2]. Our patient’s AL followed this pattern.
In conclusion, ALs are rare, cystic, benign lesions. The diagnosis is established after surgery by pathological report. Adrenalectomy is the appropriate treatment of large symptomatic ALs, while small asymptomatic cysts may be treated conservatively under observation with radiological surveillance. To date, long-term results are excellent with good prognosis.
Acknowledgments
Conflict of Interest
The authors declare that they have no conflicts of interest to report.
Abbreviations
- AL
Adrenal lymphangioma
- F
Female
- M
Male
- US
Ultrasonography
- CT
Computed tomography
- MRI
Magnetic resonance imaging
References
- 1.Ates LE, Kapran Y, Erbil Y, Barbados U, Dizdaroglou F. Cystic lymphangioma of the right adrenal gland. Pathol Oncol Res. 2005;11:242–244. doi: 10.1007/BF02893858. [DOI] [PubMed] [Google Scholar]
- 2.Ellis CL, Banerjee P, Carney E, Sharma R, Netto GJ. Adrenal lymphangioma: clinicopathologic and immunohistochemical characteristics of a rare lesion. Hum Pathol. 2011;42(7):1013–1018. doi: 10.1016/j.humpath.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Lynn RB. Cystic lymphangiomas of the adrenal associated with arterial hypertension. Can J Surg. 1965;8:92–95. [PubMed] [Google Scholar]
- 4.Gruet M, Piquard B, Ducloux M. Cystic lymphangioma of the adrenal in an African woman. Bull Soc Med Afr Noire Lang Fr. 1969;14(3):549–554. [PubMed] [Google Scholar]
- 5.Boscaino N, D’Alessandro G, Romano C, Bruzzese E, De Falco A. Study of suprarenal cysts. apropos of a case of cystic lymphagioma of the adrenal glands. Minerva Chir. 1977;32:939–946. [PubMed] [Google Scholar]
- 6.Lesobre B, Piéron R, Mafart Y, Coppin M, Horiot A, Helenon C, Favre M. Cystic lymphangioma of the adrenal gland with arterial hypertension. Sem Hop. 1977;53:377–382. [PubMed] [Google Scholar]
- 7.Georgi M, Weiss H, Trede M, Saeger HD, Bleyl U, von Mittelstaedt G. Radiologic differential diagnosis of adrenal gland cysts. Röfo. 1982;137:637–646. doi: 10.1055/s-2008-1056271. [DOI] [PubMed] [Google Scholar]
- 8.Millon G, Toulouse J, Etievent JP, Saint-Hillier Y, Perol C, Bittard M. Cystic lymphangioma of the adrenal gland causing arterial hypertension. J Urol (Paris) 1982;88:389–392. [PubMed] [Google Scholar]
- 9.Roumieu G, Panuel M, Clément JP. Adrenal cystic lymphangioma. report of a case. J Radiol. 1986;67:693–695. [PubMed] [Google Scholar]
- 10.Pfister B, Henry JF, Conte-Devolx B, Lantieri O, Codaccioni JL. Adrenal tumors of fortuitous discovery. 13 cases. Presse Med. 1987;16:1075–1078. [PubMed] [Google Scholar]
- 11.Gossot D, Decazes JM, Sarfati E, Dubost C. Cystic hemolymphangioma of the adrenal gland. J Chir (Paris) 1987;124:404–405. [PubMed] [Google Scholar]
- 12.Sanromá OI, Garrido RC, Garmendia Larrea JC, Hernández Lecuona I, Arocena Lanz F. Cystic lymphangioma of suprarenal gland. Arch Esp Urol. 1988;41:550–552. [PubMed] [Google Scholar]
- 13.Gleeson MJ, McMullin JP. Cystic lymphangiomata of the adrenal gland. Br J Urol. 1988;62:93–94. doi: 10.1111/j.1464-410X.1988.tb04281.x. [DOI] [PubMed] [Google Scholar]
- 14.Costantino V, Petrin P, Da Lio C, Zaramella D, Pedrazzoli S. Adrenal cystic masses. Our experience. Minerva Med. 1993;84:553–558. [PubMed] [Google Scholar]
- 15.Berthet B, Christophe M, Siméoni J, Jean F, Le Treut YP, Bricot R, Assadourian R. Cystic lymphangioma of the adrenal gland: three misleading cases. Presse Med. 1993;22:64–66. [PubMed] [Google Scholar]
- 16.Camara A, Guichard R, Kanane O, Toumieux B. Unilateral spontaneous hematoma of cystic lymphangioma of the adrenal gland in a pregnant woman. Presse Med. 1994;23:98–100. [PubMed] [Google Scholar]
- 17.Iderne A, Duchène H, Bruant P. Cystic lymphangioma of the adrenal gland. J Chir (Paris) 1995;132:87–89. [PubMed] [Google Scholar]
- 18.Duca S, Lupu E, Popa EL, Acalovschi I, Ban A, Medeşan R. Cystic lymphangioma of the adrenal gland. Chirurgia (Bucur) 1995;44:43–47. [PubMed] [Google Scholar]
- 19.Pérez Alapont FM, Martínez García R, Compañ Quilis A, Borrell Palanca A, García Garzón J, Gil Salom M, Garcia Sisamon F. Cystic lymphangioma of the adrenal gland in adults. Review of the literature and report of a new case. Actas Urol Esp. 1996;20:739–742. [PubMed] [Google Scholar]
- 20.Ness DT, Demeure MJ. Large lymphangioma of the adrenal gland: case report. Endocr Pract. 1996;2:245–246. doi: 10.4158/EP.2.4.245. [DOI] [PubMed] [Google Scholar]
- 21.Mortelé KJ, Hoier MR, Mergo PJ, Ros PR. Bilateral adrenal cystic lymphangiomas in nevoid basal cell carcinoma (Gorlin-Goltz) syndrome: US, CT, and MR findings. J Comput Assist Tomogr. 1999;23:562–564. doi: 10.1097/00004728-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Hoeffel CC, Kamoun J, Aubert JP, Chelle C, Hoeffel JC, Claudon M. Bilateral cystic lymphangioma of the adrenal gland. South Med J. 1999;92:424–427. doi: 10.1097/00007611-199904000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Longo JM, Jafri SZ, Bis KB. Adrenal lymphangioma: a case report. Clin Imaging. 2000;24:104–106. doi: 10.1016/S0899-7071(00)00192-3. [DOI] [PubMed] [Google Scholar]
- 24.Trojan J, Schwarz W, Zeuzem S, Dietrich CF. Cystic adrenal lymphangioma: incidental diagnosis on abdominal sonography. AJR. 2000;174:1164–1165. doi: 10.2214/ajr.174.4.1741164. [DOI] [PubMed] [Google Scholar]
- 25.Yokota M, Tanaka K, Shiotsu T, Tamura M, Nakamura S, Yamasaki Y. Lymphangiomatous adrenal cyst: a case report. Nishinihon J Urol. 2000;62:318–321. [Google Scholar]
- 26.Touiti D, Deligne E, Cherras A, Fehri HF, Maréchal JM, Dubernard JM. Cystic lymphangioma in the adrenal gland: a case report. Ann Urol (Paris) 2003;37:170–172. doi: 10.1016/S0003-4401(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 27.Satou T, Uesugi T, Nakai Y, Hayashi Y, Imano M, Hashimoto S. Case of adrenal lymphangioma with atypical lymphocytes in aspirate cytology. Diagn Cytopathol. 2003;29:87–90. doi: 10.1002/dc.10322. [DOI] [PubMed] [Google Scholar]
- 28.Luncă S, Romedea NS, Roată C, Bouras G. Cystic lymphangioma of the adrenal gland. Chirurgia (Bucur) 2004;99:255–258. [PubMed] [Google Scholar]
- 29.Robledo-Ogazón F, Vargas-Rivas AE, Alvarado-Aparicio A. Adrenal gland lymphangiomas. A case report. Cir Cir. 2004;72:213–216. [PubMed] [Google Scholar]
- 30.Garcia M, Louis LB, 4th, Vernon S. Cystic adrenal lymphangioma. Arch Pathol Lab Med. 2004;128:713–714. doi: 10.5858/2004-128-713-CAL. [DOI] [PubMed] [Google Scholar]
- 31.Nouira K, Bedioui H, Belhiba H, Daghfous A, Baccar S, Menif E, Ben Safta Z, Slim R. Cystic lymphangioma of the adrenal gland, from radiologic diagnosis to laparoscopic treatment. A case report. Tunis Med. 2007;85:160–162. [PubMed] [Google Scholar]
- 32.Bettaïeb I, Mekni A, Bédioui H, Nouira K, Chelly I, Haouet S, Bellil S, Bellil K, Kchir N, Zitouna M. Huge cystic lymphangioma of the adrenal gland. A case report and review of the literature. Pathologica. 2007;99:19–21. [PubMed] [Google Scholar]
- 33.Pereira Gallardo S, Gómez Torres FJ, Torres Olivera FJ. Cystic lymphangioma of the adrenal glands. Case report. Arch Esp Urol. 2007;60:187–189. doi: 10.4321/S0004-06142007000200011. [DOI] [PubMed] [Google Scholar]
- 34.Chien HP, Chang YS, Hsu PS, Lin JD, Wu YC, Chang HL, Chuang CK, Tsuei KH, Hsueh C. Adrenal cystic lesions: a clinocopathological analysis of 25 cases with proposed histogenesis and review of the literature. Endocr Pathol. 2008;19:274–281. doi: 10.1007/s12022-008-9046-y. [DOI] [PubMed] [Google Scholar]
- 35.Ait Ali A, Sall I, Kaoui H, Bouchentouf M, Hajjouji A, Jeddou CO, Zentar A, Sair K. Abdominal cystic lymphangioma in the adult. An exceptionally polymorphous tumor. J Afr Hepato Gastrenterol. 2009;3:7–12. doi: 10.1007/s12157-009-0063-3. [DOI] [Google Scholar]
- 36.Cutaja V, Lo Re G, Midiri M. Cystic lymphangioma of adrenal gland. Case report and review of the literature. Acta Medica Mediterranea. 2009;25:133–137. [Google Scholar]
- 37.Bisceglia M, Carosi I, Scillitani A, Pasquinelli G. Cystic lymphangioma-like adenomatoid tumor of the adrenal gland: case presentation and review of the literature. Adv Anat Pathol. 2009;16:424–432. doi: 10.1097/PAP.0b013e3181bb6c09. [DOI] [PubMed] [Google Scholar]
- 38.Sallami S, Chelly I, Nidhameddine K. Adrenal cystic lymphangioma. Tunis Med. 2013;91(1):77–78. [PubMed] [Google Scholar]
- 39.Liu B, Li Y, Wang S. Adrenal lymphangioma removed by a retroperitoneoscopic procedure. Oncol lett. 2013;5(2):539–540. doi: 10.3892/ol.2012.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthet B, Assadourian R. A complication of adrenal cystic lymphangioma: hemorrhage. Presse Med. 1989;18:939. [PubMed] [Google Scholar]
- 41.Constantino V, Pasqualli C, Liessi G. Linfangioma cistico surrenalico. Presentazione di un caso. Minerva Chir. 1992;47:1841–1845. [PubMed] [Google Scholar]
- 42.Solomou EG, Patriarheas GV, Mpadra FA, Karamouzis MV, Dimopoulos I. Asymptomatic adult cystic lymphangioma of the spleen: case report and review of the literature. Magn Reson Imag (MRI) 2003;21:81–84. doi: 10.1016/S0730-725X(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 43.Poncelet V. Retroperitoneal cystic lymphangioma. J Belge Radiol. 1998;81:245. [PubMed] [Google Scholar]
- 44.Kim JK, Yoo KS, Moon JH, Park KH, Chung YW, Kim KO, Park KH, Hahn T, Park SH, Kim JH, Jeon JY, Kim MJ, Min KS, Park CK. Gallbladder lymphangioma: a case report and review of the literature. World J Gastroenterol. 2007;13:320–323. doi: 10.3748/wjg.v13.i2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanal TH, Kocaoglu M, Yildirim D, Bulakbasi N, Guvenc I, Tayfun C, Ucoz T. Imaging features of benign adrenal cysts. Eur J Radiol. 2006;60:465–469. doi: 10.1016/j.ejrad.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Rozenblit A, Morehouse HT, Amis ES. Cystic adrenal lesions: CT features. Radiology. 1996;201:541–549. doi: 10.1148/radiology.201.2.8888255. [DOI] [PubMed] [Google Scholar]
- 47.Elsayes KM, Mukundan G, Narra VR, Lewis JS, Jr, Shirkhoda A, Farooki A, Brown JJ. Adrenal masses: MR imaging features with pathologic correlation. Radiographics. 2004;24:S73–S86. doi: 10.1148/rg.24si045514. [DOI] [PubMed] [Google Scholar]
- 48.Lubikowski J, Umiński M, Andrysiak-Mamos E, Pynka S, Fuchs H, Wójcicki M, Szajko M, Moleda P, Post M, Zochowska E, Kiedrowicz B, Safranow K, Syrenicz A. From open to laparoscopic adrenalectomy: thirty years’ experience of one medical centre. Endokrynol Pol. 2010;61:94–101. [PubMed] [Google Scholar]
- 49.Phitayakorn R, McHenry CR. Laparoscopic and selective open resection for adrenal and extraadrenal neuroendocrine tumors. Am Surg. 2008;74:37–42. [PubMed] [Google Scholar]
- 50.Zografos NG, Farfaras A, Vasiliadis G, Pappa T, Aggeli C, Vasilatou E, Kaltsas G, Piaditis G. Laparoscopic resection of large adrenal tumors. JSLS. 2010;14:364–368. doi: 10.4293/108680810X12924466007160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalof AN, Cooper K. D2-40 immunohistochemistry so far! Adv Anat Pathol. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]