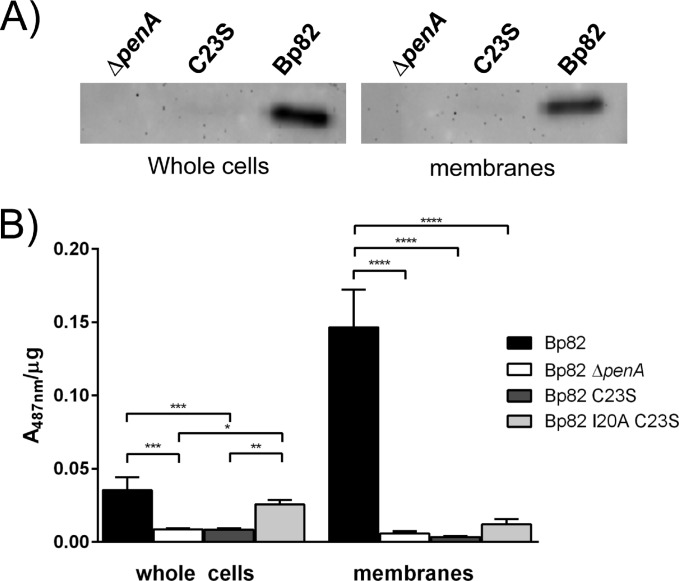
FIG 4.
Mutations of lipobox residues alter PenA stability and activity. (A) The absence of lipid modification results in the loss of detected PenA. Whole-cell extracts and total membranes of strain Bp82 (wild-type penA), Bp82 ΔpenA, and Bp82 penAC23S were analyzed by Western blotting using anti-PenA antibodies. PenA can be readily detected in strain Bp82, but not in mutants lacking PenA or expressing PenAC23S. (B) The absence of lipid modification results in the loss of PenA β-lactamase activity which can be restored by introduction of a signal peptidase I cleavage site. Nitrocefin assays were performed using whole cells and total membrane fractions of Bp82 (wild-type penA), Bp82 ΔpenA, Bp82 penAC23S, and Bp82 penAI20AC23S. Specific activity is calculated as A487 per microgram of protein. Values are shown as means plus standard errors of the means (error bars). Values that are statistically significantly different are indicated by bars and asterisks as follows: *, P ≤ 0.05, **; P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.