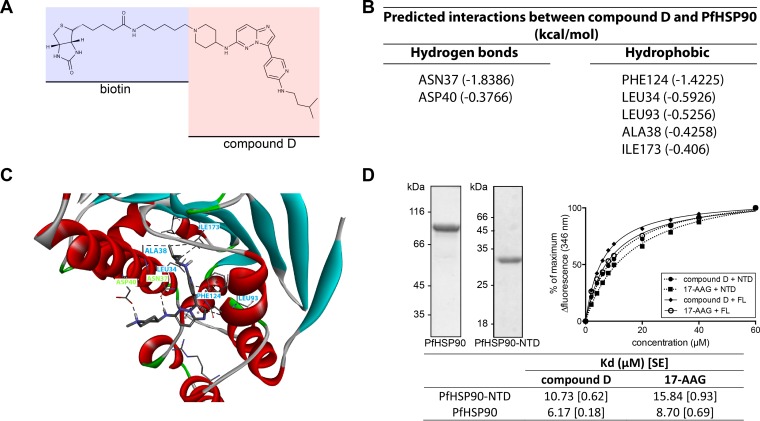
FIG 3.
Affinity purification of cellular targets of compound D. (A) Biotin linked to the R1 group of compound D. This compound was bound to streptavidin-agarose and used to affinity purify proteins from a trophozoite cell lysate. The only significant hit identified by LC-MS/MS was HSP90 (see Table S3 in the supplemental material). (B) Predicted interactions between compound D and PfHSP90. (C) Modeling of the most likely binding orientation of compound D to the ATP binding site of HSP90 was carried out using DockingServer. Residues predicted to form hydrogen bonds with compound D are labeled in green, while those predicted to form hydrophobic interactions are labeled in blue. (D) Recombinant PfHSP90 binds to compound D. Purified recombinant PfHSP90 and PfHSP90-NTD used in subsequent experiments are shown in the Coomassie-stained gels to the left of the figure. Changes in the tryptophan fluorescence (346 nm) of PfHSP90 were monitored in the presence of increasing amounts of compound D or 17-AAG. Kd values were calculated for both full-length (FL) PfHSP90 and PfHSP90-NTD. Values in brackets are the standard errors (SE) of the means of triplicate measurements.