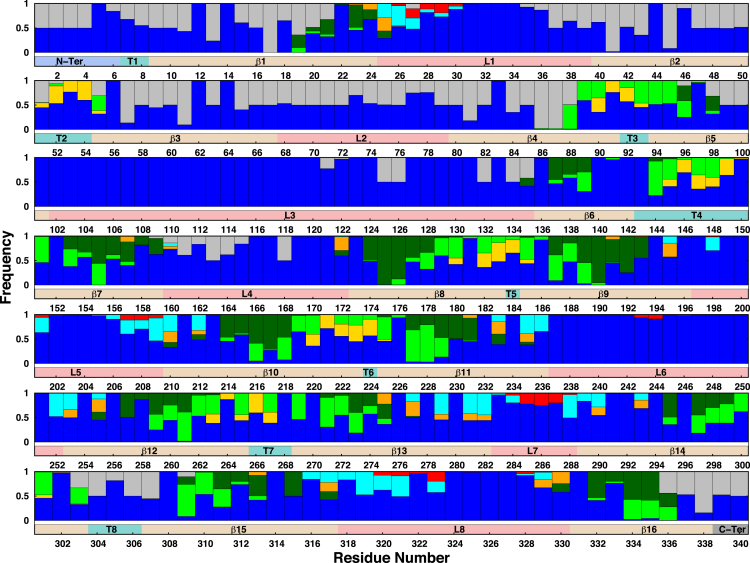
Figure 5.
Interaction patterns of OmpF protein residues with their surrounding environments in K12-lps0. The graph shows, for each residue, the frequency of interactions with another monomer (gray), water molecules (blue), a phospholipid headgroup (yellow), a phospholipid carbon tail (green), a lipid A tail (dark green), a lipid A headgroup (orange), the LPS inner core (cyan), or the LPS outer core (red). An interaction is first counted when the distance between any heavy atom of a residue and that of its interacting partner is <5 Å and is normalized for each interacting partner. The bar below each set of patterns indicates the protein secondary structure. To see this figure in color, go online.