Figure 3. Titration curves for the cysteines in the ACFCA pentapeptide.
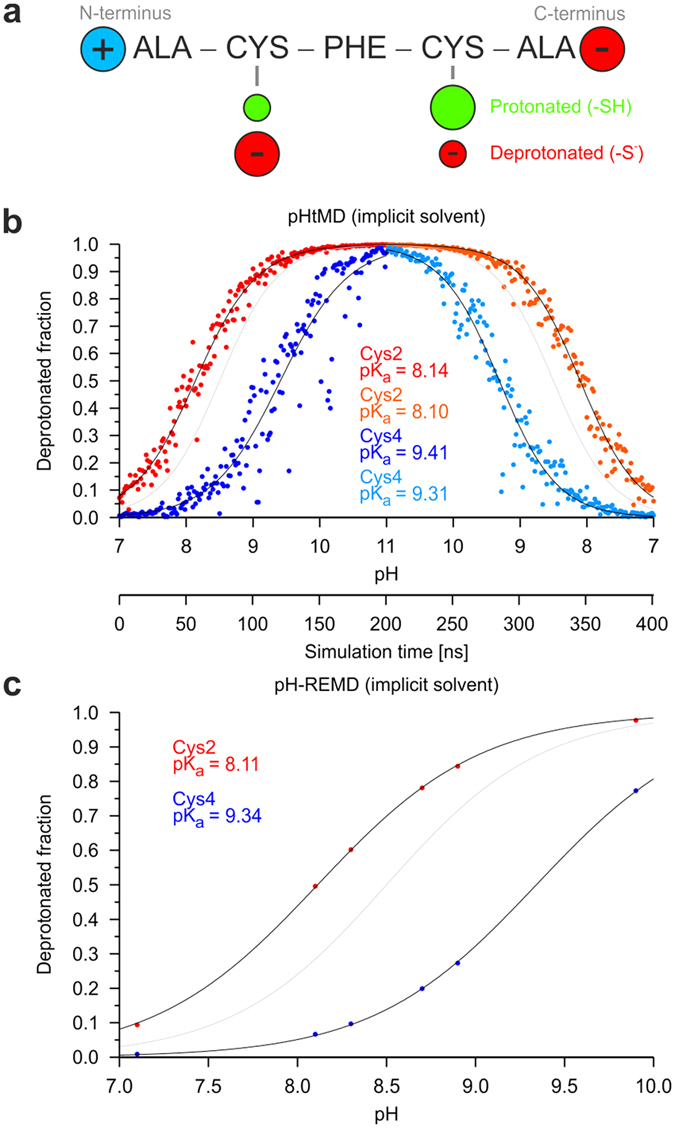
(a) In the ACFCA pentapeptide, Cys2 prefers the deprotonated, negatively charged state due to electrostatic interactions with the positively charged N-terminus. Cys4, in contrast, prefers the protonated, uncharged state due to electrostatic interactions with the negatively charged C-terminus. (b,c) Titration curves for the cysteines in ACFCA obtained from (b) a pHtMD simulation starting at pH = 7 (During the pHtMD simulation the solution pH was first increased to 11 and then again decreased to 7.) (c) a pH-REMD simulation both with implicit solvent (six replicas; each run for 10 ns). For comparison, the titration curve for the Ace-Cys-Nme is shown as a gray line.
