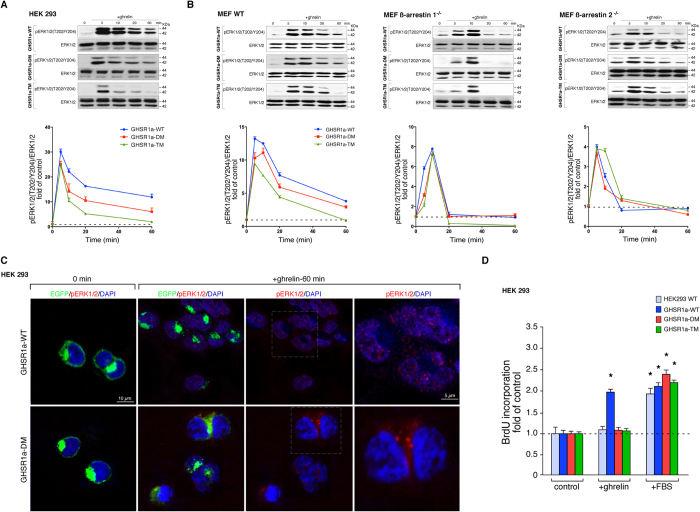
Figure 5. Functional relation between the GHSR1a-associated ß-arrestin-scaffolded complex and the ERK1/2 activity.
(A) HEK 293 cells were transiently transfected with GHSR1a-WT or mutants and stimulated with ghrelin (100 nM) for the indicated times. Samples of cell lysates were separated by SDS PAGE and immunoblots were performed using anti pERK1/2(T202/Y204) or anti total ERK1/2 antibodies. The levels of pERK1/2were quantified by densitometry, normalized to total ERK1/2 and expressed as the fold change relative to the unstimulated cells. (B) The MEF WT, ß-arrestin 1−/− and ß-arrestin 2−/− cells were treated as in (A). The levels of pERK1/2(T202/Y204) were expressed as the fold change relative to the unstimulated cells. In (A,B) immunoblots are representative of three independent experiments, the data are expressed as the mean ± SEM. (C) HEK 293 cells expressing GHSR1a-WT and GHSR1a-DM were stimulated with ghrelin for indicated time before being fixed and stained with anti pERK1/2(T202/Y204) antibodies, images were acquired by confocal microscopy. DAPI was used as a counterstain to identify nuclei and is shown in blue. Confocal images are representative of three independent experiments. (D) Mitogenic effect of ghrelin (100 nM) on cells transiently transfected with the GHSR1a-WT or mutants (n = 6). Results were expressed as a fold increase in BrDU incorporation relative to control cells. The data are expressed as the mean ± SEM (*,#p < 0.05).