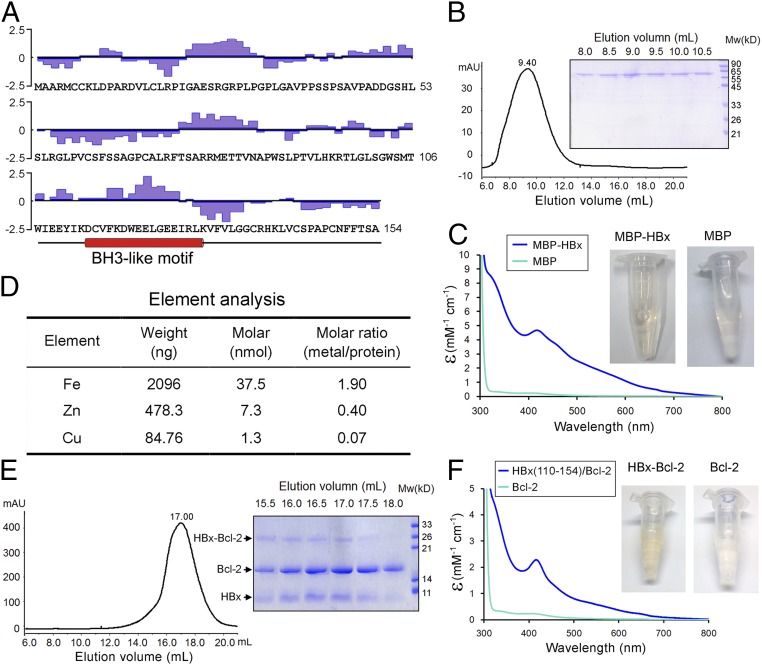
Fig. 1.
Biochemical characterization of the hepatitis B virus protein HBx. (A) The primary sequences of HBx. The hydrophilicity plot by the Kyte–Doolittle criteria (48) is shown above the sequences. The 63 hydrophobic amino acids (Ala, Val, Leu, Ile, Phe, Cys, and Met) account for 41% of the total. In addition, there are 15 Pro and 4 Trp residues. The putative BH3-like motif is shown below the sequences in an α-helical conformation. (B) The full-length HBx appears to form large oligomers on gel filtration. The full-length MBP-tagged HBx was eluted from gel filtration with an elution volume of 9.4 mL, close to the void (Left). The peak fractions were visualized on SDS/PAGE (Right). (C) The purified full-length MBP-tagged HBx exhibits a yellow-brownish color. Wavelength scan of this protein, but not the MBP control, shows an absorption peak at 415 nm. (D) Element analysis of the full-length MBP-tagged HBx reveals the presence of metal ions. Both iron and zinc are present in significant quantities with ∼1.9 and 0.4 molar equivalence of the MBP-HBx protein. (E) The HBx fragment (residues 110–154) bound to Bcl-2 exhibits excellent behavior on gel filtration. The fusion protein between the HBx fragment and Bcl-2 (residues 1–50 and 92–207) was purified to homogeneity and separated by proteolysis. The resulting complex was subject to gel filtration analysis (Left). The peak fractions were visualized on SDS/PAGE (Right). (F) The HBx fragment (residues 110–154) bound to Bcl-2 has a yellow-brownish color. Wavelength scan of HBx-Bcl-2 shows an absorption peak at 415 nm.