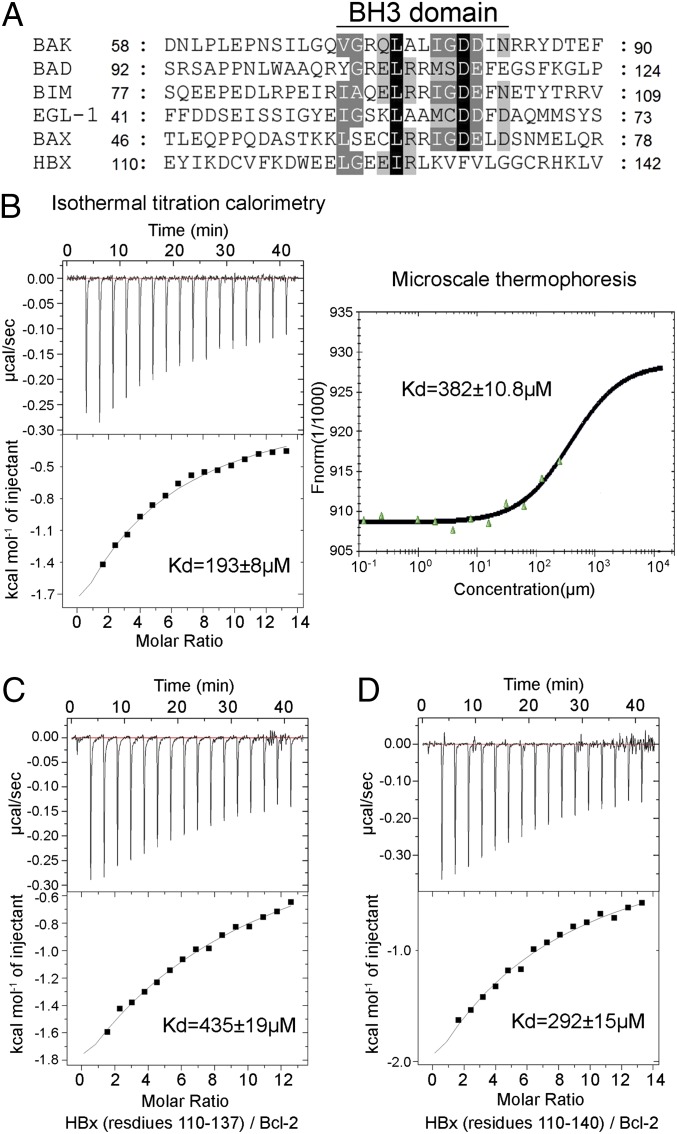
Fig. 2.
Quantification of the interactions between the HBx BH3-like motif and Bcl-2. (A) Sequence alignment of the BH3 domains from the proapoptotic Bcl-2 proteins and HBx. Invariant and conserved residues among the canonical BH3 motifs are shaded black and gray, respectively. (B) Measurement of binding affinities between the HBx BH3-like motif (residues 110–135) and Bcl-2 (residues 1–50 and 92–207). Measurement by ITC and MST reveals dissociation constants of 193 ± 8 and 382 ± 10.8 µM, respectively. (C) Measurement of the binding affinity between the HBx fragment (residues 110–137) and Bcl-2 by ITC. Data fitting reveals a dissociation constant of 435 ± 19 µM. (D) The HBx fragment (residues 110–140) binds to Bcl-2 with a dissociation constant of ∼292 ± 15 µM by ITC.