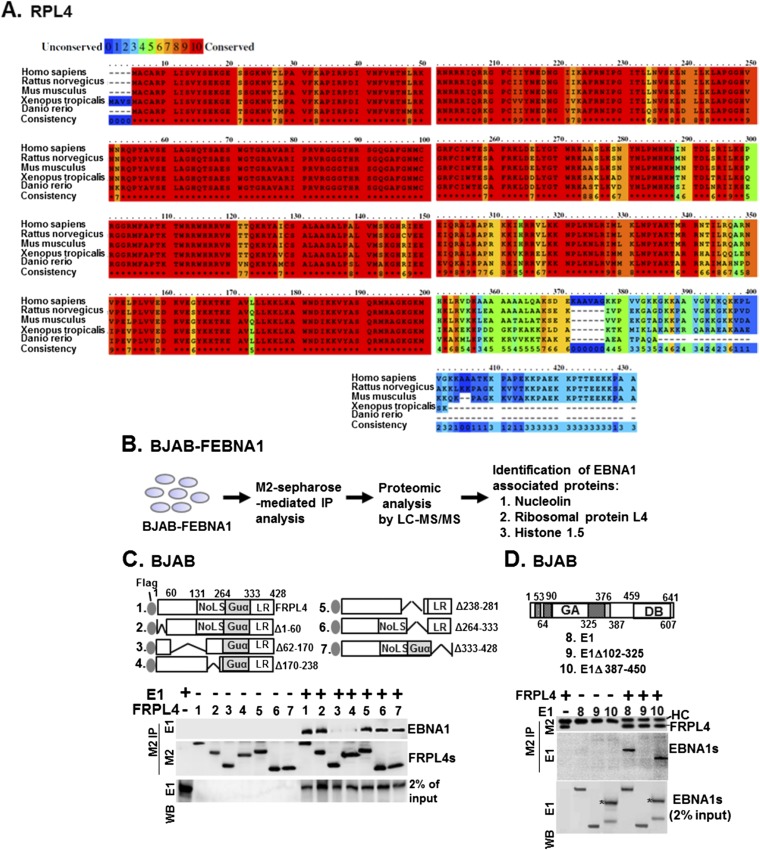
Fig. S1.
Mapping of the protein–protein interacting domain of EBNA1 and RPL4. (A) Sequence alignments of RPL4 from human, rat, mice, Xenopus, and zebra fish were shown. The degrees of related amino acid sequence conservation were indicated by numbers 0 (none) to 10 (high). (B) The flowchart of the previously described proteomic procedure for identification of EBNA1-associated proteins by LC/MS-MS (18). Both NCL and RPL4 were found to be EBNA1-associated. (C) Transfection-mediated co-IP assays were used to identify the EBNA1 binding domain of RPL4 using plasmids of FRPL4 or each of its mutant derivatives, with E1 cotransfection. We loaded 2% input of EBNA1. (D) The reciprocal experiment of C was conducted using plasmids of E1 or the indicated deletion mutants with cotransfected FRPL4. The corresponding band produced by E1 387–450 was marked with an asterisk. HC, heavy chain.