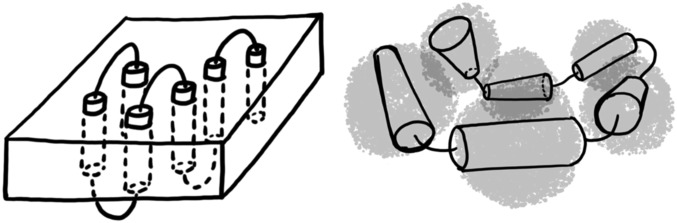
Fig. 1.
Schematic diagrams of the unfolded state of α-helical membrane proteins in bilayers (Left) and detergent micelles (Right). The transmembrane helices (cylinders) are connected by loops. Transmembrane helices are either embedded in a membrane (rectangular prism) or are surrounded by detergent micelles (transparent gray spheres). In this work, we use an implicit membrane model to simulate folding within a bilayer and assume that folding in detergent micelles corresponds to folding without constraints on the alignment of helices. In both cases, we assume that the unfolded state has near-native levels of secondary structure, as has been observed in experiments on the SDS-denatured state of membrane proteins.