Abstract
Background & Aims
It is important to identify superficial (T1) gastroesophageal adenocarcinomas (EAC) that are most or least likely to metastasize to lymph nodes, to select appropriate therapy. We aimed to develop a risk stratification model for metastasis of superficial EAC to lymph nodes using pathologic features of the primary tumor.
Methods
We collected pathology data from 210 patients with T1 EAC who underwent esophagectomy from1996 through 2012 on factors associated with metastatsis to lymph nodes (tumor size, grade, angio-lymphatic invasion, and submucosal invasion). Using these variables, we developed a multivariable logistic model to generate 4 categories for estimated risk of metastasis (<5% risk, 5%–10% risk, 15%–20% risk, or >20% risk). The model was validated in a separate cohort of 39 patients who underwent endoscopic resection of superficial EAC and subsequent esophagectomy, with node stage analysis.
Results
We developed a model based on 4 pathologic factors that determined risk of metastasis to range from 2.9% to 60% for patients in the first cohort. In the endoscopic resection validation cohort, higher risk scores were associated with increased detection of lymph node metastases at esophagectomy (P=.025). Among patients in the first cohort who did not have lymph node metastases detected before surgery (cN0), those with high risk scores (>20% risk) had 11-fold greater odds for having lymph node metastases at esophagectomy compared to patients with low risk scores (95% confidence interval, 2.3–52 fold). Increasing risk scores were associated with reduced patient survival time (P<.001) and shorter time to tumor recurrence (P<.001). Patients without lymph node metastases (pT1N0) but high risk scores had reduced times of survival (P<.001) and time to tumor recurrence (P=.001) after esophagectomy than patients with pT1N0 tumors and lower risk scores.
Conclusions
Pathologic features of primary superficial EACs can be used, along with the conventional node staging system, to identify patients at low risk for metastasis, who can undergo endoscopic resection, or at high risk, who may benefit from induction or adjuvant therapy.
Keywords: prognostic, lymphatic invasion, tumor budding, tumor grade, submucosal invasion
Introduction
In the majority of patients, surgically resected, superficial (T1) adenocarcinoma of the esophagus or gastroesophageal junction (EAC) has a favorable survival outcome relative to more deeply invasive cancers.[1] However, despite tumor that is confined to the mucosal or submucosal layers , up to 16% of patients with T1 EAC will have nodal metastases identified at surgical resection.[2-7] These patients have significantly worse prognosis.[3, 8] Currently, decisions for therapeutic intervention are based primarily on estimation of risk for nodal metastasis using depth of tumor invasion and clinical assessment of nodal stage. For patients with T1 cancers thought to have minimal risk of nodal metastases, many centers are advocating endoscopic resection (ER) alone, or less extensive nodal dissection during esophagectomy. Conversely, patients thought to have nodal metastasis based on clinical staging are often referred for induction chemo-radiotherapy. Unfortunately, endoscopic ultrasound (EUS), while more sensitive than either computed tomography (CT) or 18F-fluoro-2-deoxy-D-glucose positron emission tomography (PET), is only 70-80% sensitive and specific for nodal metastasis.[9] Complementary methods must be employed to accurately estimate the likelihood of nodal metastases.
Based on a widespread consensus in the literature,[2-7, 10-14] submucosal invasion is routinely evaluated by staging ER of superficial EAC and is regarded as the paramount risk factor for nodal metastasis.[15] However, we and others have identified additional histopathologic risk factors for nodal metastasis, including angiolymphatic invasion, [5, 6, 11, 13-17] higher grade, [4-6, 11, 14, 15, 17] tumor budding[17] and larger tumor size.[5, 11, 13, 15, 17] It is standard practice for pathologists to report some or all of these findings in pre-operative staging ER specimens. Hence, in practice, clinicians must decide how best to treat tumors with knowledge of all of these characteristics. In spite of the widely held belief that invasion into the submucosa is a “watershed” for nodal metastasis,[15] there appears to be a trend towards the treatment of both intramucosal and submucosally invasive adenocarcinomas by ER.[18] This may be due to other clinical considerations (e.g. patient age and comorbidities) or the perception that it is possible to identify submucosal cancers with an acceptably low risk of nodal metastasis.[19]
The central aim of this study is to develop a quantitative model of the probability of nodal metastasis based on rigorously defined, known pathologic risk factors and evaluate how it may complement current methods of risk stratification based on clinical node staging. We also sought to determine whether the presence of histopathologic risk factors in the primary tumor would be associated with survival outcome, particularly in cases pathologically staged as node negative (pT1N0) that are unlikely to receive adjuvant treatment.
Methods
Patient/Case Selection
This study was approved by the University of Pittsburgh Institutional Review Board with waiver of consent. We reviewed all patients with superficial (T1) EAC recorded who underwent esophagectomy from 1996 to 2012. Patients with high grade dysplasia only, patients initially treated by ER and patients who received neoadjuvant therapy were excluded. The cases included in this study have been previously reported. [17] The electronic medical record and physical charts were reviewed for patient- and treatment- variables. Pre-operative clinical stage was assigned based on radiographic findings (including available CT, PET and/or PET/CT scans) as well as EUS examination.
A total of 210 patients had representative slides available for detailed histopathologic review. Without knowledge of the pathologic lymph node stage, all available diagnostic hematoxylin and eosin stained slides representing the primary tumor were reviewed by the authors (ML and JD) in order to score the following pathologic features of the primary tumor: depth of invasion, tumor grade, angiolymphatic invasion and tumor size (scoring criteria are summarized in Table 1 as previously described [17]). The final classification of each variable was based on the consensus diagnosis of the two pathologists. After scoring these pathologic features, the originally reported lymph node stage was confirmed on histologic review.
Table 1.
Scoring Histopathologic Risk Factors for Nodal Metastasis
| Risk Factor | Classification or Measurement Criteria* |
|---|---|
|
Depth of Invasion Intramucosal (T1a) Superficial T1a Deep T1a Submucosal (T1b) Superficial T1b Deep T1b |
EAC invading no deeper than the true muscularis mucosae EAC confined to the lamina propria EAC invading any layer of the muscularis mucosae (duplicated or true) EAC invading into the submucosa EAC invading no deeper than the upper half of the submucosa, assessed at the deepest point of invasion EAC invading into the lower half of the submucosa, assessed at the deepest point of invasion |
|
Histologic Grade Low Grade High Grade |
Well or moderately differentiated (>50% tubular, papillary or gland forming; based on all tumor sections) AND no more than focal tumor budding Poorly differentiated (<50% tubular, papillary or gland forming; based on all tumor sections) OR extensive tumor budding |
|
Angiolymphatic Invasion Present Absent |
Unequivocal evidence of tumor epithelial cells within endothelium lined vascular space Above criterion not met |
| Tumor Size | Tumor size (in cm) based on maximal cross sectional dimension on histologic sections or maximal gross tumor size measurement; for multifocal tumors, size of the largest focus was used; tumors were then classified as < 2cm or ≥ 2 cm |
As previously described in Landau et al. [26]
For survival analysis, overall survival was defined as the time interval from esophagectomy to all-cause death and censored at the date of last contact. Time to first recurrence was defined as the time interval from esophagectomy to first clinical diagnosis of recurrence and censored at the time of last clinical evaluation for recurrence. Local (anastomotic, gastric, esophageal), regional nodal as well as distant metastatic recurrences were included.
Statistical Analysis
Variables were characterized descriptively using medians and the inter-quartile range (IQR) for continuous variables or categories and percentages for discrete variables. Significance of differences was assessed with the Wilcoxon rank-sum test for continuous variables and either chi-square test or Fisher's exact test for categorical variables. Significance of associations with the outcome of nodal metastases were first evaluated using a univariate logistic model. Clinically relevant pathologic variables were all included the multivariable model. The resulting model coefficients were applied to the cohort to calculate predicted values from the logistic equation, i.e. ŷ = 1/(1 + exp[−(Xβ)]. Additional details concerning model development and evaluation are reported as Supplementary Information.
The survival estimates for each predicted risk group were characterized using Kaplan-Meier curves, with statistical differences tested using the log-rank test after excluding patients who died peri-operatively (survival <3 months). P<0.05 was considered statistically significant. Analysis was performed using SPSS version 22 (IBM, Armonk, NY).
Validation Cohort
We identified a separate cohort of 163 patients with T1a or T1b EAC diagnosed by ER of the primary tumor in our institution from 2006-2014, of which 43 underwent subsequent esophagectomy with lymph node staging. The remaining cases were followed by serial EUS and/or PET/CT for nodal metastasis or recurrence. We validated the model in patients who underwent esophagectomy by extracting depth of invasion, tumor size, tumor grade and angiolymphatic invasion from the pathologic reports and assigning them to a risk group based on the model coefficients. In our institution during this time period, EMR specimens were diagnosed by subspecialist GI pathologists with synoptic reporting of the risk factors and regular consensus review.
Results
Clinical and Pathologic Characteristics
The majority of patients in the study cohort were males with Barrett's-associated cancers located at the gastroesophageal junction and treated by minimally invasive esophagectomy. (Table 2) Forty-one patients (19%) had at least one lymph node metastasis at esophagectomy. The final pre-operative node stage was cN1 in 23% of patients based on the comprehensive pre-operative evaluation for regional nodal disease that included, at a minimum, a CT of the chest and abdomen for assessment of enlarged lymph nodes. Additional clinical staging parameters used in other patients include PET scan and EUS. (Table 2)
Table 2.
Clinical and Pathologic Characteristics of the Study Cohort
| All Cases (N=210) | Node Negative (N=169) | Node Positive (N=41) | P value | |
|---|---|---|---|---|
| Average Patient Age, years | 67 | 67 | 67 | 0.866 |
| Sex, N (%) | ||||
| Male | 179 | 143 (80) | 36 (20) | 0.606 |
| Female | 31 | 26 (84) | 5 (16) | |
| Tumor Location, N (%) | ||||
| Esophagus | 85 | 72 (85) | 13 (15) | 0.202 |
| Gastroesophageal Junction | 125 | 97 (78) | 28 (22) | |
| Barrett's Esophagus, N (%) | ||||
| Present | 193 | 160 (83) | 33 (17) | 0.007 |
| Absent | 17 | 9 (53) | 8 (47) | |
| Operative Approach, N (%) | ||||
| Minimally invasive esophagectomy | 180 | 145 (81) | 35 (19) | 0.943 |
| Other (hybrid, open) | 30 | 24 (80) | 6 (20) | |
| Clinical Staging Modality, N (%) | ||||
| Endoscopic ultrasound | 160 | 131 (82) | 29 (18) | 0.294 |
| Computed tomography scan | 210 | 169 (80) | 41 (20) | 1.000 |
| PET scan | 82 | 65 (79) | 17 (21) | 0.712 |
| Pre-operative Clinical N Stage, N (%) | ||||
| Negative (cN0) | 158 | 138 (87) | 20 (13) | <0.001 |
| Positive (cN1) | 49 | 28 (57) | 21 (43) | |
| Unknown (cNx) | 3 | |||
| Pathologic Tumor Stage (AJCC7) , N (%) | ||||
| Intramucosal | 72 | 69 (96) | 3 (4) | <0.001 |
| Submucosal | 138 | 100 (72) | 38 (28) | |
| Histologic Tumor Grade, N (%) | ||||
| Low Grade | 142 | 124 (90) | 14 (10) | <0.001 |
| High Grade | 68 | 41 (60) | 27 (40) | |
| Angiolymphatic Invasion, N (%) | ||||
| Absent | 174 | 150 (86) | 24 (14) | <0.001 |
| Present | 36 | 19 (53) | 17 (47) | |
| Tumor Size, N (%) | ||||
| <2 cm | 105 | 97 (92) | 8 (8) | <0.001 |
| ≥2 cm | 105 | 72 (69) | 33 (31) | |
| Pathologic Node Stage (AJCC7) , N (%) | ||||
| pN0 (0 positive lymph nodes) | 169 | 169 (100) | -- | N/A |
| pN1 (1-2 positive lymph nodes) | 26 | -- | 26 (100) | |
| pN2 (3-6 positive lymph nodes) | 10 | -- | 10 (100) | |
| pN3 (>6 positive lymph nodes) | 5 | -- | 5 (100) | |
| Lymph nodes examined, median (IQR) | 20 (13-28) | 20 (13-28) | 19 (14-28) | 0.969 |
Association of Individual Pathologic Characteristics with Nodal Metastasis
Nodal metastasis were found in 27.5% of patients with submucosal invasion (pT1b) compared to 4.2% of patients with intramucosal adenocarcinoma (pT1a), an 8.7-fold increase in the odds of nodal metastasis (95% confidence interval 2.6-29.5 fold, P<0.001, Table 3). All 3 node positive pT1a cases in our study had a single nodal metastasis and no recurrences were seen in 3 to 10 years of follow up. Although the entire visible lesion was evaluated in each case (ranging in size from 0.8 to 2.1 cm), we cannot entirely exclude understaging given the retrospective nature of the study. The odds of nodal metastasis were also significantly increased, ranging from 5.6- to 6.0-fold, in the presence of high tumor grade, angiolymphatic invasion and tumor size larger than 2.0 cm. (Table 3)
Table 3.
Univariate and Multivariate Association of Individual Pathologic Variables with Nodal Metastasis at Esophagectomy
| Univariate (Unadjusted) Odds Ratio (95% CI) | Multivariate (Adjusted) Odds Ratio (95% CI) | |
|---|---|---|
| Pathologic Tumor Stage | ||
| Intramucosal, pT1a | Ref | Ref |
| Submucosal, pT1b | 8.7 (2.6-29.5) | 2.6 (0.7-13.5) |
| Histologic Tumor Grade | ||
| Low Grade | Ref | Ref |
| High Grade | 6.0 (2.8-12.6) | 3.1 (1.4-7.0) |
| Angiolymphatic Invasion | ||
| Absent | Ref | Ref |
| Present | 5.6 (2.6-12.2) | 2.5 (1.0-6.2) |
| Tumor Size | ||
| <2 cm | Ref | Ref |
| ≥2 cm | 5.6 (2.4-12.8) | 2.2 (0.9-4.9) |
Multivariable Analysis of Pathologic Characteristics’ Association with Nodal Metastasis
In the multivariable logistic regression model that included all four pathologic characteristics known to be significantly associated with lymph node metastasis from the univariate analysis, only high tumor grade remained an independent predictor of nodal metastasis. (Table 3) Other pathologic factors clearly modify the risk of nodal metastasis associated with depth of invasion: compared to intramucosal (T1a) lesions, submucosal (T1b) EAC were significantly larger (median 2.5 cm vs. 0.9 cm, P< 0.001), more likely to be high grade (45.7% vs. 6.9%, P<0.001) and have angiolymphatic invasion (25.4% vs. 1.4%, P<0.001).
Estimating the Probability of Nodal Metastasis Using the Multivariable Logistic Regression Model and Comparison to Depth of Invasion Alone
In general, the model predicts that as more risk factors are identified on histologic examination, there will be a progressive increase in the probability of nodal metastasis, though each factor is weighted individually. (Supplementary Information and Table 4) The model generated probability estimates ranging from 2.9% (T1a, < 2 cm, low grade and no angiolymphatic invasion) up to 60% (T1b, ≥ 2cm, high grade with angiolymphatic invasion). The estimated probability of lymph node metastasis was calculated for each patient and the ROC curve generated. The model yielded an area under the ROC curve (AUROC) of 0.801 (95% confidence interval, 0.726-0.876) for predicting nodal metastasis. (Supplementary Figure 1) By comparison, a logistic model of metastatic risk using only depth of invasion (stratified into superficial and deep T1a, superficial and deep T1b, criteria described in Table 1) yielded an AUROC of 0.718 (95% confidence interval, 0.639-0.797) for predicting nodal metastasis. (Supplementary Figure 2)
Table 4.
Tumor Characteristics and Estimated Probability of Nodal Metastasis with Observed Rate of Nodal Metastasis
| Tumor Characteristics | Model Estimated Probability of Nodal Metastasis | Observed Rate of Nodal Metastasis |
|---|---|---|
| T1a with no other risk factors | Very low (<5%) | 2/57 (3.5%) |
| T1a with 1 or more risk factors* | Estimated >5%* | 1/15 (6.7%)* |
| T1b with no other risk factors | Low (5-10%) | 2/27 (7.4%) |
| T1b with ALI OR >2cm alone | Intermediate (15-20%) | 6/38 (15.8%) |
| T1b with high grade alone | High (~20%) | 3/13 (23.1%) |
| T1b with 2 or more additional risk factors** | High (30-60%) | 27/60 (45.0%) |
Most tumors in this category had a single risk factor (tumor size > 2 cm, N=10; high grade, N=3) and would have a low (5-10%) estimated probability. T1a cancers with two risk factors were rare (N=2); estimated probability in this group was intermediate (15-20%), which could not be confirmed due to the rarity of these cases.
These tumors invaded the submucosa and had at least 2 additional risk factors (ALI, high tumor grade and/or tumor size > 2cm)
Tumor size, angiolymphatic invasion and tumor grade define a spectrum of risk of nodal metastasis in submucosal EAC
Submucosal (T1b) EAC had a significantly higher rate of nodal metastasis in comparison to T1a EAC (Table 3), but the presence or absence of other risk factors defined a spectrum of risk among tumors that invaded into the submucosa. (Table 4) Despite having the “high risk” characteristic of submucosal invasion, the estimated probability of nodal metastasis generated by the model assigned 27 T1b EAC to the “low risk” category. Only 2 of these patients had pathologic nodal metastasis (7.1%; Table 4). In contrast, when the model yielded a high estimated probability of nodal metastasis, 30 of 73 (41%) T1b EAC had pathologically confirmed nodal metastasis (P<0.001).
The large majority (79%) of intramucosal (T1a) EACs fell into the very low risk group (tumor < 2cm in size, low grade and no angiolymphatic invasion), while the remaining 21% of T1a EAC had one or two pathologic risk factors that predicted a higher risk of nodal metastases of at least 5%. (Table 4) However, the observed rate of nodal metastasis in these two groups was not significantly different (P=0.509) and the magnitude of the estimated risk should, therefore, be interpreted with caution.
Validation of the model risk estimates in endoscopically resected EAC with subsequent esophagectomy
We identified a total of 163 T1a or T1b EAC that underwent attempted ER of the primary tumor with clinical (by serial EUS and/or PET/CT) or pathologic evaluation for metastasis. Pathologically confirmed nodal metastases were diagnosed in 3/107 (2.8%) patients with T1a EAC (1 diagnosed at esophagectomy; 2 on fine needle aspiration, FNA) and 8/56 (17.0%) T1b EAC (7 at esophagectomy, 1 on FNA).
Thirty-nine of 43 patients subsequently treated by esophagectomy were included in the validation set and assigned to a risk group based on documentation of pathologic risk factors. Two patients were excluded because they were node negative after induction therapy. Two were excluded due to undocumented tumor size. None of these cases were included in the development of the risk model. Pathologic features, risk group assignment, and indication for esophagectomy are summarized in Supplementary Table 1. Nodal metastases were identified in 1/17 (5.9%) cases with <10% estimated risk; in 3/13 (23.1%) cases with 15-20% estimated risk; and 4/9 (44.4%) cases with 30-60% estimated risk (P = 0.025).
Discordance between estimated pathologic risk and pre-operative clinical stage identifies cases with incorrect pre-operative clinical staging
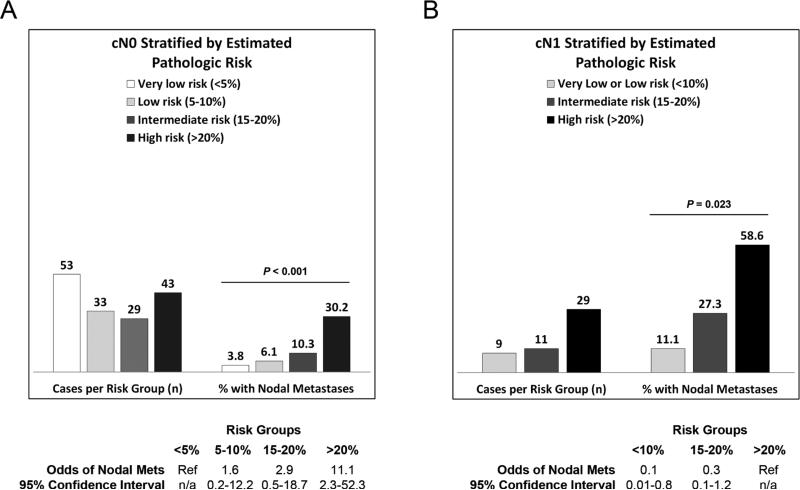
Pre-operative clinical node staging by EUS and other imaging studies was inaccurate in 23% (48/207) of patients based on comparison to the final pathologic node stage. In the clinically N0 patients, when the probability of nodal metastasis generated by the model was estimated to be high (>20%), there was an 11-fold increased odds of pathologically confirmed nodal metastasis. (Figure 1A) Similarly, in the clinically node positive (cN1) group, a low (<10%) estimated risk of nodal metastasis was associated with a 90% reduction in the odds of nodal metastasis. (Figure 1B)
Figure 1.
Observed rate of nodal metastasis at esophagectomy in patients with a negative (cN0) and positive (cN1) pre-operative staging evaluation for nodal metastasis after stratification by estimated probability of nodal metastasis.
We defined pathologic risk/clinical node staging discordance as: >20% pathologic risk and cN0; or <10% pathologic risk and cN1. When pathologic risk and clinical node stage were concordant, the clinical node stage agreed with the final pathologic node stage in a significantly higher proportion of cases [82.6% (128/155) versus 59.6% (31/52), P=0.001].
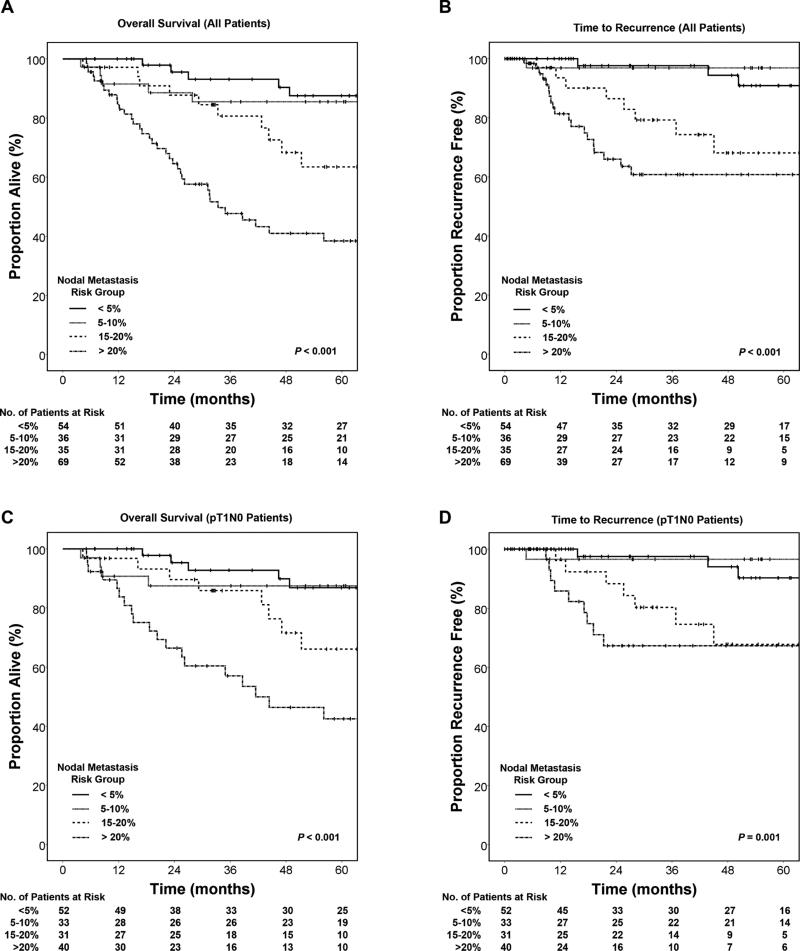
A high estimated pathologic risk of nodal metastasis is associated with worse survival and higher rate of recurrence, including the subset of patients staged as pT1N0 at esophagectomy
Patients were stratified into four risk groups (very low, <5%; low, 5-10%; intermediate, 15-20%; and high, >20%) and analyzed for overall survival and time to recurrence outcomes via Kaplan-Meier plots. Increasing estimated risk of nodal metastasis had a significant adverse effect on both overall survival (Figure 2A, P<0.001) and time to recurrence (Figure 2B, P<0.001).
Figure 2.
Survival functions stratified by model estimated probability of nodal metastasis.
For the subset of patients pathologically staged as node negative (pT1N0) at esophagectomy, there was a similar adverse effect on survival (Figure 2C, P<0.001) and recurrence (Figure 2D, P=0.001). In this subset, the average lymph node count did not significantly differ between cases with low or very low risk (21 lymph nodes/esophagectomy) and cases with intermediate to high risk features (22 lymph nodes/esophagectomy, P=0.327). The pattern of recurrence was similar as well: there were distant metastases in 13 of 16 recurrent intermediate to high risk and 3 of 4 recurrent low or very low risk pT1N0 cases. Pathologic risk estimate remained a significant predictor of overall mortality (P=0.002) and time to recurrence (P=0.009) in the pT1N0 subgroup after adjusting for patient age, number of lymph nodes examined and type of operative procedure.
Discussion
The aim of this study was to develop a useful prediction model for nodal metastasis in T1 esophageal adenocarcinoma that integrates known pathologic risk factors into a more nuanced description of metastatic risk and extends the much relied upon binary risk classification (i.e. intramucosal = low risk; submucosal = high risk). Our study has several strengths, including the large size of the model development cohort, which enabled multivariable prediction modeling, a rigorous definition of pathologic variables based on dedicated review, and validation of the model in a separate cohort of patients that underwent ER followed by esophagectomy.
A previous study by Lee et al. suggested that depth of invasion, tumor size, ALI and grade might be combined into a multivariable risk assessment.[20] However, because this study was based only on data from pathology reports from multiple institutions, the histologic criteria used to assess the risk factors could not be defined.
Our multivariable approach to risk assessment shows that some submucosal EAC have a low (5-10%) risk of lymph node metastasis due to the absence of other risk factors, while others have significantly higher (30-60%) risk. Our results echo the study of Manner et al. who reported that only 1 of 53 (1.9%) patients with T1b EAC and “low risk” pathologic characteristics developed a nodal metastasis after ER of the primary tumor and complete endoscopic remission.[21] The model does not differentiate between superficial submucosal and deep submucosal invasion, though we [17] and others [4, 11, 14, 15] have previously found that deep invasion of the submucosa carries a greater risk of nodal metastasis than invasion of the superficial submucosa, though not all studies are in agreement on this.[5, 6, 10, 16] Others recommend measuring the linear depth of invasion into the submucosa.[22] It is possible that further refinement of the depth of invasion would improve the prediction model, but it would require a considerably larger cohort of cases to assess this question in sufficient detail. We felt that a cutoff of any submucosal invasion may be more easily replicated, especially in ER specimens, because of several factors that may preclude accurate assessment of depth of invasion: (1) inherent irregularity of the muscularis mucosae (the upper border of the submucosa); (2) obliteration of the muscularis mucosae by tumor; (3) suboptimal tissue orientation.
The model has potential applications in various clinical settings when considering the need for esophagectomy with lymphadenectomy or the need for systemic therapy. Our findings support the routine use of staging ER of superficial cancers as a complement to pre-operative clinical lymph node staging. Most patients with a discrepancy between pathologic risk and clinical staging in our study were understaged. Discrepant results warrant careful review of pre-operative staging to ensure its accuracy. Further study is required to determine if this improves preoperative nodal staging accuracy.
For patients who are deemed poor operative candidates and who undergo ER of a superficial cancer with features indicating a high risk of nodal metastasis, our results suggest that use of adjuvant systemic therapy may warrant consideration. Although the model was developed to predict nodal metastases, our data suggest that it may also be a useful indicator of long-term outcome, even among patients staged pT1N0 (Stage I) at esophagectomy, among whom we identified a subset with high risk pathologic features and relatively poor survival outcomes who may therefore benefit from adjuvant treatment. Additional studies to determine whether there is a survival benefit to such an approach are needed.
We acknowledge that a model based on esophagectomy specimens has limitations when applied in the setting of endoscopic treatment. A primary concern is the inherent inaccuracy of retrospective pathologic examination of esophagectomy specimens. It may be noted that the proportion of T1a cancers with nodal metastasis (4.2%) in the model development cohort is higher than many surgical series, raising concern about the accuracy of staging depth of invasion. [23] We found a similar, but somewhat lower rate of pathologically confirmed nodal metastasis (2.8%) in a separate cohort of carefully staged T1a EAC diagnosed by ER in our institution. This rate of nodal metastasis in our ER cohort is higher than the rate estimated by Pech et al.[24] There are several factors that may have contributed to this discrepancy: (1) we excluded cases with no invasion of the lamina propria (“intraepithelial adenocarcinoma”); (2) we did not exclude clinically node positive patients from either cohort; (3) our patients were evaluated by serial EUS and PET/CT for nodal metastasis; (4) some regional metastatic disease may be clinically indolent or elicit an anti-tumor immune response that effectively neutralizes the tumor, precluding detection on routine clinical follow-up; (5) selection bias resulting in a disproportionate number of high risk cases treated at our institution. These considerations notwithstanding, the data from other studies, [23, 24] supported by our data, indicate that T1a lesions without other histologic risk factors pose a very low metastatic risk and low risk of cancer-associated death. Because of the small number of T1a EAC with other risk factors and the low rate of metastasis in this group, our estimates of metastatic risk at the lowest end of the risk spectrum should be regarded with caution.
In contrast, the independently validated model does strongly suggest that T1b EAC can be risk stratified into low, intermediate and high risk groups with significantly different rates of nodal metastasis at esophagectomy. In the model development cohort, we included some large, submucosal lesions that, in all likelihood, would not be considered endoscopically resectable, but did influence the estimate of metastatic risk in the model development cohort. Tumors that are endoscopically resectable are node negative by clinical staging parameters and relatively superficial. Studies that include only ER specimens may have lower estimates of metastatic risk. In patients with T1b EAC that underwent attempted ER in our institution, only 17% had nodal metastasis, compared to 28% of the T1b EAC that underwent primary esophagectomy. However, the validation cohort confirmed that there is a subset of T1b EAC with a significantly higher risk of nodal metastasis that can be identified by histologic examination of ER specimens. Even so, the model estimates should be prospectively validated.
In conclusion, assessing these four variables may yield a more robust, risk-adjusted approach to surgical and medical therapy decisions for superficial EAC in the pre-operative setting (e.g. at the time of staging ER) and in the post-operative setting.
Supplementary Material
Acknowledgements
The authors wish to thank Patrice Gibbs, MS (statistician, Center for Research on Health Care Data Center), for her efforts in conducting many of the preliminary statistical analyses under the direction of Dr. Landsittel.
Grant Support: The project was supported in part by award number K07CA151613 (KSN) from the National Cancer Institute. The project was also supported by the National Institutes of Health through Grant Number UL1TR000005.
Abbreviations
- ALI
angiolymphatic invasion
- AUROC
area under the receiver operating characteristic curve
- CI
confidence interval
- CT
computed tomography
- EAC
esophageal adenocarcinoma
- ER
endoscopic resection
- EUS
endoscopic ultrasound
- IQR
inter-quartile range
- PET
positron emission tomography
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Authors have no conflicts of interest to disclose.
Author Contributions: JMD (study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; statistical analysis; study supervision); MSL (acquisition of data; critical revision of the manuscript); JDL (study concept; acquired funding; critical revision of the manuscript); KMM (critical review of the manuscript; study concept); TJF (acquisition of data); DPL (statistical analysis; critical revision of the manuscript); MKG (material support; acquired funding; critical revision of the manuscript); KSN (study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; acquired funding; statistical analysis; study supervision)
References
- 1.Rice TW, et al. Cancer of the esophagus and esophagogastric junction. Cancer. 2010;116(16):3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 2.van Sandick JW, et al. Pathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction: implications for therapeutic decision making. Cancer. 2000;88(11):2429–37. doi: 10.1002/1097-0142(20000601)88:11<2429::aid-cncr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122(6):1077–90. doi: 10.1067/mtc.2001.113749. [DOI] [PubMed] [Google Scholar]
- 4.Westerterp M, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446(5):497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 5.Leers JM, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253(2):271–8. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 6.Barbour AP, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol. 2010;17(9):2494–502. doi: 10.1245/s10434-010-1025-0. [DOI] [PubMed] [Google Scholar]
- 7.Stein HJ, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242(4):566–73. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holscher AH, et al. Early adenocarcinoma in Barrett's oesophagus. Br J Surg. 1997;84(10):1470–3. [PubMed] [Google Scholar]
- 9.van Vliet EP, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98(3):547–57. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollschweiler E, et al. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38(2):149–56. doi: 10.1055/s-2006-924993. [DOI] [PubMed] [Google Scholar]
- 11.Buskens CJ, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60(5):703–10. doi: 10.1016/s0016-5107(04)02017-6. [DOI] [PubMed] [Google Scholar]
- 12.Cen P, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer. 2008;112(5):1020–7. doi: 10.1002/cncr.23265. [DOI] [PubMed] [Google Scholar]
- 13.Ruol A, et al. Prevalence, management and outcome of early adenocarcinoma (pT1) of the esophago-gastric junction. Comparison between early cancer in Barrett's esophagus (type I) and early cancer of the cardia (type II). Dis Esophagus. 1997;10(3):190–5. doi: 10.1093/dote/10.3.190. [DOI] [PubMed] [Google Scholar]
- 14.Estrella JS, et al. Duplicated muscularis mucosae invasion has similar risk of lymph node metastasis and recurrence-free survival as intramucosal esophageal adenocarcinoma. Am J Surg Pathol. 2011;35(7):1045–53. doi: 10.1097/PAS.0b013e318219ccef. [DOI] [PubMed] [Google Scholar]
- 15.Raja S, et al. Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg. 2011;142(6):1403–11 e1. doi: 10.1016/j.jtcvs.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Badreddine RJ, et al. Depth of Submucosal Invasion Does Not Predict Lymph Node Metastasis and Survival of Patients With Esophageal Carcinoma. Clinical Gastroenterology and Hepatology. 2010;8(3):248–253. doi: 10.1016/j.cgh.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau MS, et al. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkow RP, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106(7) doi: 10.1093/jnci/dju133. [DOI] [PubMed] [Google Scholar]
- 19.Manner H, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc. 2014 doi: 10.1007/s00464-014-3881-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee L, et al. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg. 2013;217(2):191–9. doi: 10.1016/j.jamcollsurg.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Manner H, et al. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early Stage Adenocarcinoma of the Esophagus With Low-risk sm1 Invasion. Clin Gastroenterol Hepatol. 2013;11(6):630–5. doi: 10.1016/j.cgh.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Eguchi T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol. 2006;19(3):475–80. doi: 10.1038/modpathol.3800557. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012;107(6):850–62. doi: 10.1038/ajg.2012.78. quiz 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pech O, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652–660 e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.