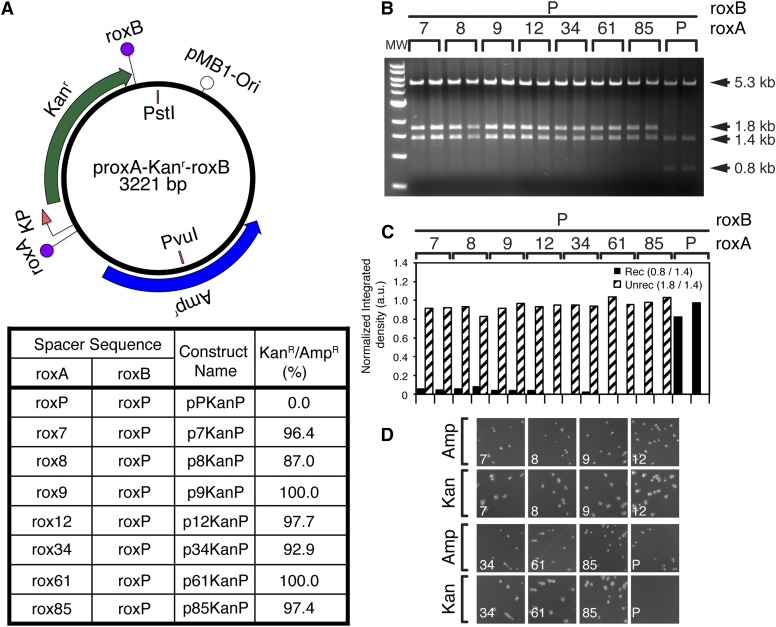
Figure 3.
Identified rox mutants are indeed unable to recombine with the wild type. Seven of the identified mutant spacers (A) were tested for the ability to recombine with the wild type roxP site, by cotransformation with pGB2-Dre in DH5α. Transformations were plated on ampicillin+ spectinomycin+, and two minipreps for each clone were amplified and diagnosed by restriction digest with PvuI + PstI (B, C). In addition, both clones were retransformed into DH5α plated on ampicillin plates, and replica plated using a velvet replicator onto kanamycin plates (D). (A) Schematic of the generic recombination target construct (top), and naming convention for the individual mutant constructs (bottom table). The roxB site is always roxP. The roxA site is either a wild type rox site (pPKanP) or one of the seven mutant spacers (Figure 2E). (B) Digest with PvuI + PstI. The 5.3 kb band represents the linearized pGB2-Dre. Both isolates of all seven mutant clones failed to recombine (1.8 + 1.4 kb bands), while the wild type isolates fully recombined (1.4 + 0.8 kb fragments). (C) Densitometric analysis confirms minimal recombination for mutants 7–9, and essentially no recombination for mutants 12–85. (D) Replica plating experiments for one of the two isolates for each clone, as well as the wild type control. For each clone, the number is indicated in the bottom left corner. Top rows are initial ampicillin plates, and bottom rows replicates onto kanamycin plates. The ratio of colonies surviving on kanamycin vs. ampicillin plates is shown as percent in A, bottom table. Images of full plates are provided in Figure S2.