Figure 4.
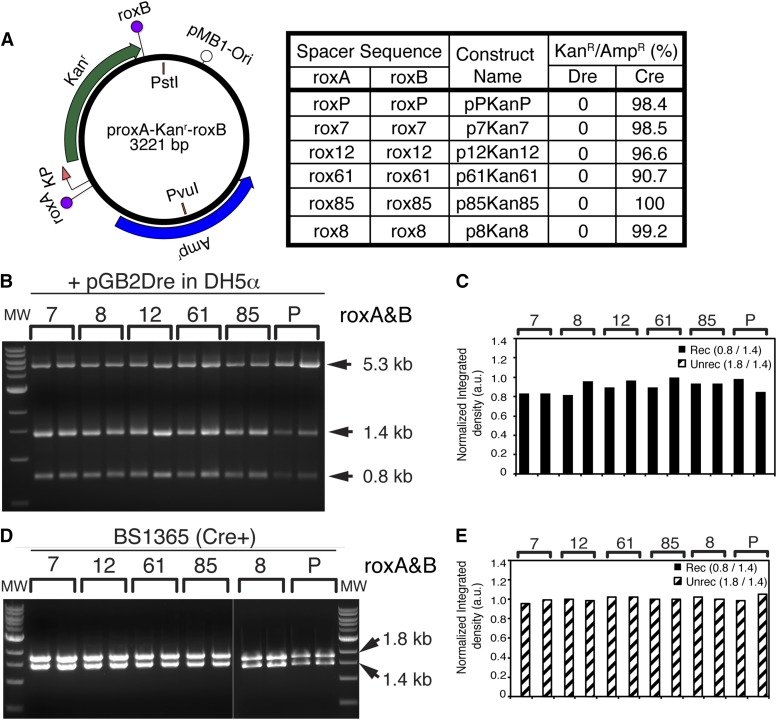
Rox mutants rox7, rox8, rox12, rox61, and rox85 recombine with themselves in the presence of Dre but not Cre recombinase. (A) Rox sites with spacers identical to clones 7, 8, 12, 61, and 85 (Figure 2E) were cloned at both the roxA and roxB positions in direct orientation, generating the p7Kan7, p8Kan8, p12Kan12, p61Kan61, and p85Kan85 constructs. Each construct, as well as the pPKanP positive control, were then exposed to Dre recombinase by the same cotransformation protocol outlined in Figure 3. Two separate isolates for each clone were then tested by PvuI + PstI restriction digestion (B, C) and replica plating (Figure S3, A, B, C, and F). All rox sites tested showed complete recombination (1.4 + 0.8 kb fragments) when exposed to Dre recombinase, and essentially no surviving colonies when replica plated from ampicillin to kanamycin plates (Figure S3A, and table in A, right hand column). Quantitations of B are shown in C. To test the sensitivity of the wild type roxP and mutants to Cre recombination, pPKanP and p7Kan7, p8Kan8, p12Kan12, p61Kan61, and p85Kan85 mutants were transformed in the BS1365 strain, constitutively expressing Cre. Transformations were plated on kanamycin and ampicillin, and two individual colonies from each clone were amplified and analyzed by PvuI + PstI restriction digestion (D, E). In addition, DNA from the two colonies was retransformed in DH5α, plated on ampicillin, and colonies replica plated onto kanamycin plates using a velvet replicator (Figure S3B). All tested constructs show the 1.8 and 1.4 kb fragments characteristic for lack of recombination (D) and resistance to kanamycin in the replica platting assay (Figure S3B and table in A, right hand column). Note that the BS1365 strain carries the Cre recombinase on a F’ element that also contains a kanamycin resistance gene. Therefore, kanamycin does not select against rox target vector recombinants in the initial BS1365 cotransformation step.