Figure 1.
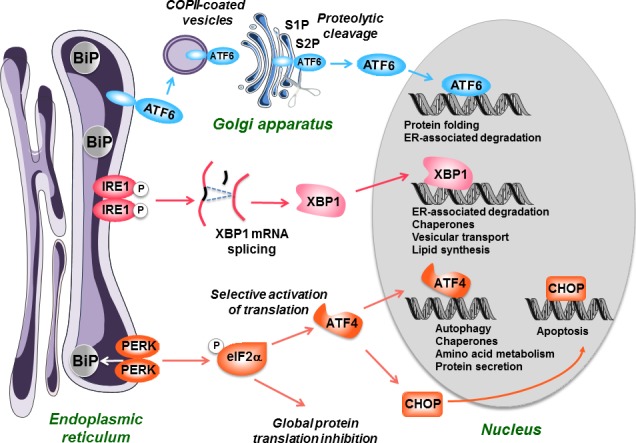
The unfolded protein response (UPR). When an endoplasmic reticulum (ER) stress occurs, the cell initiates an adaptive response called the UPR. It starts with the activation of three effectors, PKR‐like ER kinase (PERK), IRE1, and ATF6, following the removal of the chaperone BiP (GRP78) that maintains them in an inactivated state. PERK is a kinase which phosphorylates and inactivates the elongation initiation factor eIF2α, leading to a general decrease in protein translation. However, eIF2α selectively stimulates the translation of ATF4, a transcription factor which possesses a specific structure (uORF) on its mRNA. ATF4 then activates the synthesis of chaperones and proteins involved in autophagy, protein secretion, and amino acid metabolism. IRE1 possesses a kinase activity leading to its autophosphorylation and activation of a RNAse activity. This leads to the splicing of XBP1 mRNA, which is then translated into an active transcription factor. The transcription factor ATF6, which is bound to the ER membranes as an inactive precursor is transferred via COPII‐coated vesicles to the Golgi apparatus, where it is cleaved by the S1P and S2P proteases into an active form. XBP1 and ATF6 will then activate in the nucleus the transcription of a set of factors allowing to restore ER homeostasis including chaperones, foldases, and proteins involved in the degradation of unfolded polypeptides (ER‐associated degradation). If these mechanisms are not efficient to restore ER and cell homeostasis, the UPR will eventually activate mechanisms leading to cell apoptosis, in particular via the transcription factor C/EBP homologous protein (CHOP).
