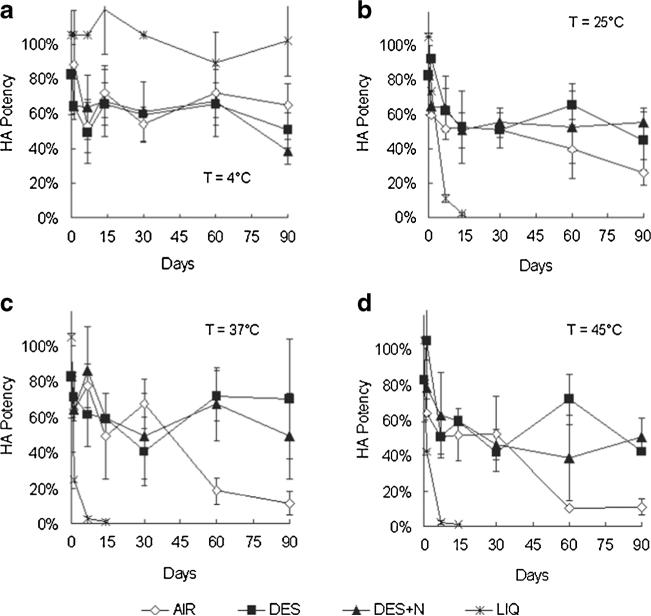
Fig. 4.
Three-months stability of inactivated influenza vaccine as a function of storage temperature, desiccation and oxygen content. Unprocessed vaccine solution was stored in sealed microcentrifuge tubes (LIQ). Microneedle patches containing vaccine were packaged in: open containers subject to ambient humidity (AIR); sealed containers containing air in the head space and desiccant to maintain low humidity (DES); and sealed containers filled with nitrogen gas in the headspace and desiccant (DES+N). Samples were stored for up to 90 days at 4°C (a), 25°C (b), 37°C (c) and 45°C (d). Data show average±s.d. (n≥3).