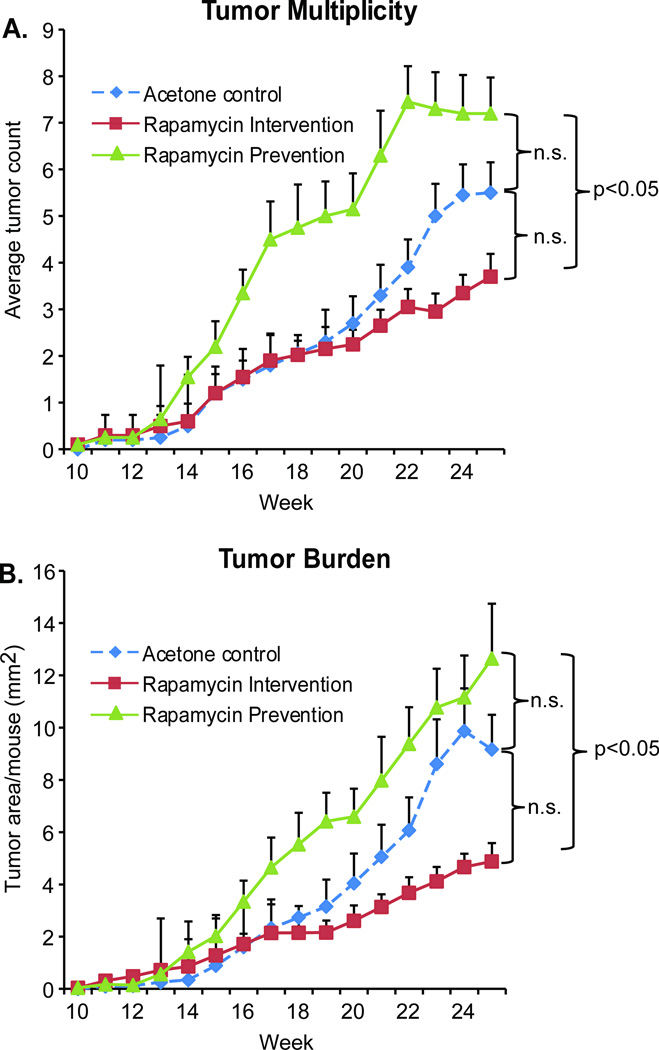
Figure 2. Treatment schedule of topical rapamycin application affects the outcome of solar simulated light-induced skin tumorigenesis.
SKH-1 hairless female mice were treated with solar simulated light (SSL) 3 times a week for 15 weeks (n = 20). Control mice were treated on their back skin with vehicle (acetone) 1hr prior to each UV exposure, and continued with 3× weekly acetone treatments after UV stopped. Mice in the “Prevention” group were treated 3 times a week with rapamycin (50nmol/back) 1 hr prior to UV exposure, and continued receiving this treatment after SSL stopped. Mice in the “Intervention” group received only vehicle (acetone) 1hr prior to each SSL treatment, but switched to treatments with rapamycin after SSL stopped. All mice were observed for tumor number and size until sacrifice at week 25, at which point tissues were harvested. Tumor multiplicity (average number of tumors per mouse) is shown in A. Tumor burden (average area per mouse) is shown in B. Data are shown as mean values + SEM.