Essential thrombocythemia (ET) is rare in children, with an annual incidence of ~100-fold lower than that in adults.1 The rarity of the disease in children makes the clinical course and pathogenesis of childhood ET far less clear. It is reported that clonal markers are much less common in children with ET (25.8%) than that in adult cases (80–90%).2, 3, 4 Except for JAK2 V617F, and MPL and calreticulin (CALR) mutations, no other mutations have yet been reported in childhood ET.2, 5, 6, 7, 8 More molecular markers are needed to distinguish clonal from reactive thrombocytosis in children. The present study investigated the JAK2 V617F, and MPL and CALR mutations in a large cohort of children with ET. We conducted the first study to analyze the molecular profiles by targeted next-generation sequencing and to investigate the JAK2 46/1 haplotype in childhood ET.
Sixty-three children diagnosed with sporadic ET according to the 2008 World Health Organization criteria were enrolled.9 Bone marrow histology was consistent with a diagnosis of ET in all cases. The molecular patterns were evaluated before any cytoreductive drug use. The JAK2 V617F, and MPL and CALR mutations were investigated as previously reported.4 The JAK2 46/1 haplotype (rs12340895) was assessed by Sanger sequencing. Fifty-five genes (Supplementary Table 1) associated with myeloid malignancies were analyzed by targeted sequencing in 25 children. Polymorphisms in existing database were excluded. Mutations were validated by Sanger sequencing (Supplementary Table 2). Each true-positive mutation was further investigated in 100 normal controls. Germline DNA was used to identify somatic mutations. Detailed information is shown in the Supplementary Methods.
The median age was 11 years (range, 3–14 years). Different from adult ET,10 a male preponderance was observed among childhood patients, with a male/female ratio of 1.5 (38/25). Compared with the adult patients that we previously reported,10 childhood ET had higher platelet counts (median 1224 × 109 vs 900 × 109/l; P<0.001), lower hemoglobin level (median 127 vs 137 g/l; P<0.001) and comparable white blood cell counts (median 10.6 × 109 vs 9.9 × 109/l; P=0.312). It indicates a pronounced and isolated megakaryocyte proliferation in child patients. The JAK2 V617F mutation was found in 14 children (22.2%), with a median allele frequency of 22% (range, 10–31%). In 49 patients among whom CALR and MPL mutations were investigated, only an 11-year-old girl harbored a CALR mutation (52-bp deletion), and none had MPL mutations. The molecular markers were much less common than that in adult patients.3, 4 In adult patients, compared with patients with wild-type JAK2, V617F-mutated patients display higher white blood cell counts, higher hemoglobin level but lower platelet counts.4 However, there were no differences in blood cell counts between children with and without the JAK2 V617F mutation (Supplementary Table 3).
Three children (4.8%) displayed major thrombosis, and two of them were V617F-mutated. Microvascular disturbances were more common (n=30, 47.6%) than that in Caucasian children (30.3%).2 Headache was the most frequent symptom (n=22, 34.9%). The incidence of headache was significantly related to platelet counts at diagnosis (P=0.013). Headache was relieved by antiplatelet and/or cytoreductive therapy in 17 children (77.3%), providing important evidence for the central role of platelets in the etiology of headache. No major bleeding events were observed. The rate of minor bleeding episodes was 14.3% (n=9), similar to that in the Caucasian children (9.0%).2 The risk of minor bleeding events was not related to platelet counts (P=0.126) or JAK2 V617F mutation (P=0.194)). Two children (3.2%) evolved to myelofibrosis after 20 and 7 years of follow-up, respectively. One harbored the JAK2 V617F mutation and received 1 year of intermittent hydroxyurea treatment before transformation. The other one did not have JAK2 V617F or CALR mutations, and received 2 months of interferon-alpha and then 5 years of hydroxyurea before transformation.
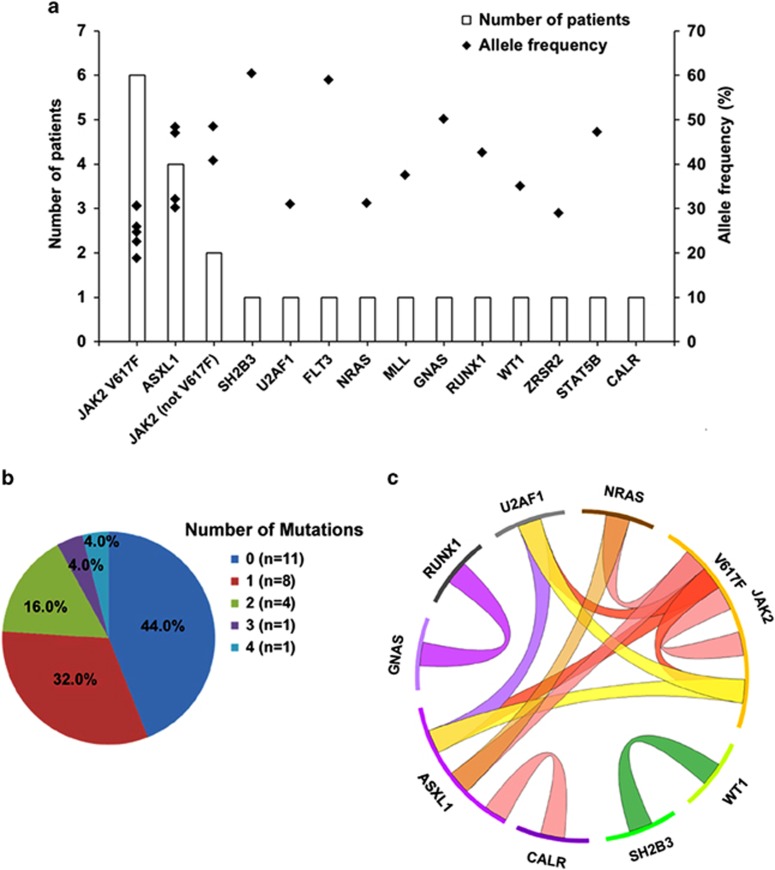
Targeted sequencing was performed in 25 children (Supplementary Table 4). The average depth (median, 349-fold) and coverage (median 99.6%) of the target regions were excellent (Supplementary Figure 1). A total of 135 single-nucleotide variants were identified (Supplementary Table 5). A detailed filtration pipeline was developed to select mutations that might be related to tumorigenesis (Supplementary Figure 2). After filtration, eighteen types of somatic mutations (single nucleotide variants and indels) involving 13 genes and 7 germline mutations involving 7 genes were selected (Supplementary Table 6). Somatic mutations were present in 14 children (56.0%), with a median allele frequency of 33.6% (range, 18.8–60.4%). Other than JAK2 V617F (n=6, 24.0%), the most frequently observed somatic mutations were ASXL1 mutations (n=4, 16.0%) (Figure 1a). In 18 children with wild-type JAK2 and CALR, seven (38.9%) harbored somatic mutations that were previously undocumented in childhood ET. Six (24.0%) children harbored two or more somatic mutations (Figure 1b).
Figure 1.
Frequency and distribution of somatic mutations in children with essential thrombocythemia. (a) Number of patients and allele frequency of each mutated gene. (b) Number of patients with different number of somatic mutations. (c) Co-occurrence of the somatic mutations in the same individual.
The molecular profiles were different between childhood and adult ET patients. On the one hand, mutations that were commonly involved in adult ET were not found in childhood ET. In adult patients, other than JAK2, CALR and MPL, mutated genes most commonly include TET2 (4%–11%) and DNMT3A (1–5%).11 However, none of the children had TET2 or DNMT3A mutations. On the other hand, the newly identified mutations in childhood ET (that is, mutations in the gene NRAS, MLL, U2AF1, ZRSR2, GNAS, FLT3, RUNX1 and WT1) were rarely seen in adult ET but were recurrent in myelodysplastic syndrome, primary myelofibrosis or blast phase myeloproliferative neoplasms.11, 12, 13 The rate of the ASXL1 mutations was much higher (16%) than that in adult ET (2–5%).11 The discovery of recurrent mutations indicates that targeted sequencing can be used to distinguish clonal from reactive thrombocytosis in children.
By analyzing co-occurrence of the somatic mutations (Figure 1c), we revealed a genetic complexity in childhood ET. Lundberg et al.14 reported that in 60 adults with somatic mutations, 17 (28.3%) had two or more mutations. In our study, about half of the children with somatic mutations (6/14, 42.9%) had more than one mutation. The higher rate of co-occurrence of rare somatic mutations in childhood ET suggests that children with ET might have a more complex and unstable genetic composition than adult patients have. Acquisition and accumulation of the somatic mutations in early life might be the main reason for the early onset of ET in children. Mutual exclusivity was observed in gene pairs with similar biological function, such as genes both involved in the JAK–STAT pathway. However, co-occurrence of mutations in different alleles of JAK2 (that is, JAK2 V617F and JAK2 I354T; JAK2 V617F and JAK2 G127D) was observed in two children, indicating that the JAK2 gene might be more sensitive to the genetic instability. This might be one of the reasons why JAK2 was most commonly affected in ET. The definite existence of genetic instability needs more evidence.
The JAK2 46/1 haplotype was assessed in 49 children. The number of children with CC, CG and GG genotypes was 17 (34.7%), 31 (63.3%) and 1 (2.0%), respectively. The frequency of the JAK2 46/1 haplotype (33.7%) was significantly higher than that in normal Chinese population (21.9% P=0.013).15 It suggests that inherited predisposition may also exist in childhood patients.
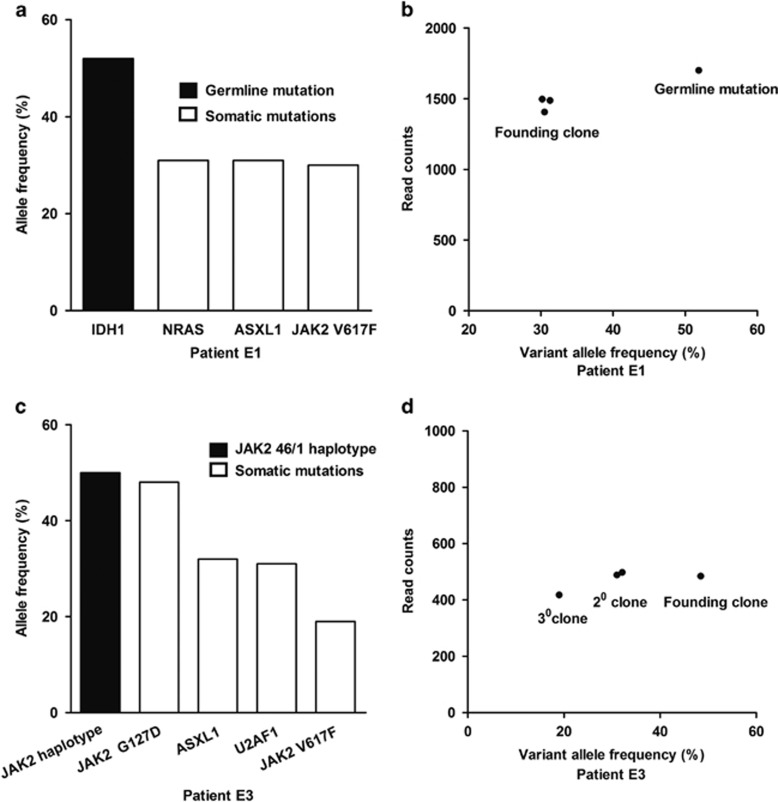
Clusters of mutations based on allele frequencies distinguish founding clones from subclones.12 Figure 2 shows diverse clonal hierarchies in two representative children. The germline IDH1 mutation in patient E1 and the JAK2 46/1 haplotype in patient E3 reflected the inherited genetic background. In patient E1, three variants (that is, NRAS, ASXL1 and JAK2 V617F) with similar allele frequencies (~30%) might exist in the same founding clone or in separate founding clones (Figures 2a and b). Different from patient E1, patient E3 had the founding clone (that is, JAK2 G127D), first-level subclones (that is, ASXL1 and U2AF1), and second-level subclone (that is, JAK2 V617F) (Figures 2c and d). Since the heterozygous JAK2 G127D mutation (allele frequency, 48.5%) was present in almost all cells, JAK2 V617F (allele frequency, 19.0%) was not the earliest genetic alteration. Similarly, the CALR mutation was also not the initial abnormality, which could be speculated from the co-occurrence of the heterozygous ASXL1 M1096L mutation (allele frequency, 48.4%) and the CALR mutation (allele frequency, 21.1%) in the same individual.
Figure 2.
Clonality assessment in two representative cases of essential thrombocythemia. (a) Allele frequencies of mutations in patient E1. (b) Clusters of variants identify the founding clone in patient E1. (c) Allele frequencies of mutations in patient E3. (d) Clusters of variants identify the founding clone and subclones in patient E3. In (b) and (d), the allele frequency is plotted versus the total number of sequencing reads covering the corresponding mutated nucleotide.
Concerning gender, age and blood cell counts, no differences were found between mutated and non-mutated children, except for white blood cell counts (P=0.025). The only child with thrombosis among the 25 children had a single ASXL1 mutation. The types of antiplatelet and cytoreductive treatment between mutated and non-mutated children were similar (Supplementary Table 4).
In conclusion, the molecular profiles are different between childhood and adult ET patients, and the genetic composition in childhood ET may be more complex than that in adults. The difference in clinical and hematological characteristics between childhood and adult ET may be due to the different molecular profiles underlying the two entities. Additional molecular markers are found by targeted sequencing to identify children with a clonal blood disorder. After JAK2 V617F, the most frequently observed somatic mutations are ASXL1 mutations. The JAK2 V617F or CALR mutations may not be the initial abnormalities in some children. Whole genome sequencing would help to reveal initial molecular events.
Acknowledgments
This study was supported by 863 projects of the Ministry of Science and Technology of China (2012AA02A211) (LZ), and National Natural Science Foundation of China (81270595 and 81470302) (LZ). English editing service was provided by Editage (http://www.editage.cn/).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Hasle H. Incidence of essential thrombocythaemia in children. Br J Haematol 2000; 110: 751. [DOI] [PubMed] [Google Scholar]
- Randi ML, Geranio G, Bertozzi I, Micalizzi C, Ramenghi U, Tucci F et al. Are all cases of paediatric essential thrombocythaemia really myeloproliferative neoplasms? Analysis of a large cohort. Br J Haematol 2015; 169: 584–589. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Xuan M, Zhou Y, Sun T, Bai J, Cao Z et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014; 28: 1912–1914. [DOI] [PubMed] [Google Scholar]
- Giona F, Teofili L, Capodimonti S, Laurino M, Martini M, Marzella D et al. CALR mutations in patients with essential thrombocythemia diagnosed in childhood and adolescence. Blood 2014; 123: 3677–3679. [DOI] [PubMed] [Google Scholar]
- Langabeer SE, Haslam K, McMahon C. CALR mutations are rare in childhood essential thrombocythemia. Pediatr Blood Cancer 2014; 61: 1523. [DOI] [PubMed] [Google Scholar]
- Kucine N, Chastain KM, Mahler MB, Bussel JB. Primary thrombocytosis in children. Haematologica 2014; 99: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zhang L, Yang R. Paediatric essential thrombocythaemia: clinical and molecular features, diagnosis and treatment. Br J Haematol 2013; 163: 295–302. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008; 22: 14–22. [DOI] [PubMed] [Google Scholar]
- Fu R, Xuan M, Lv C, Zhang L, Li H, Zhang X et al. External validation and clinical evaluation of the International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in a large cohort of Chinese patients. Eur J Haematol 2014; 92: 502–509. [DOI] [PubMed] [Google Scholar]
- Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010; 24: 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013; 122: 3616–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 2011; 364: 2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014; 123: 2220–2228. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hu T, Wu Z, Kang Z, Liu W, Guan M. The JAK2 46/1 haplotype is a risk factor for myeloproliferative neoplasms in Chinese patients. Int J Hematol 2012; 96: 611–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.