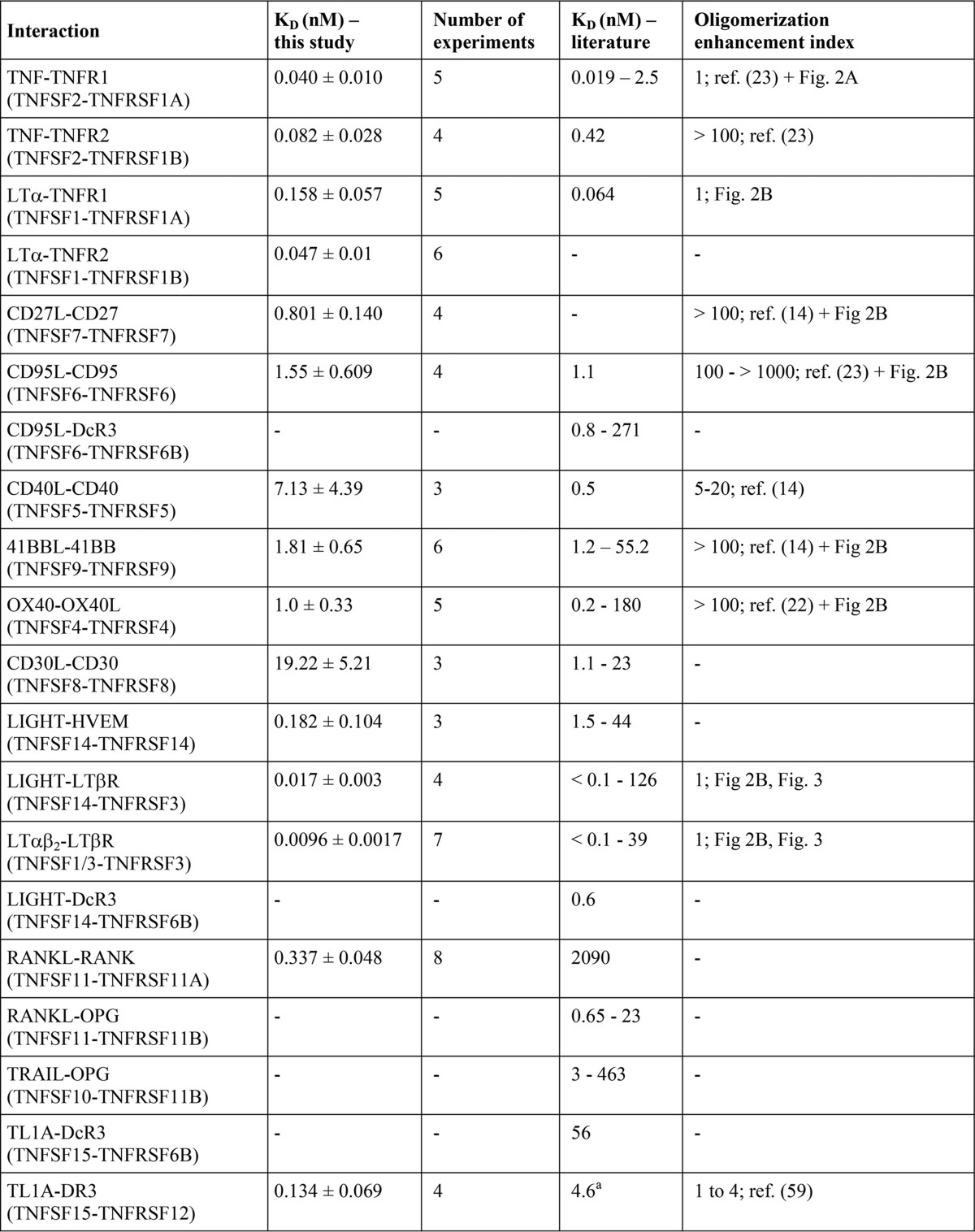
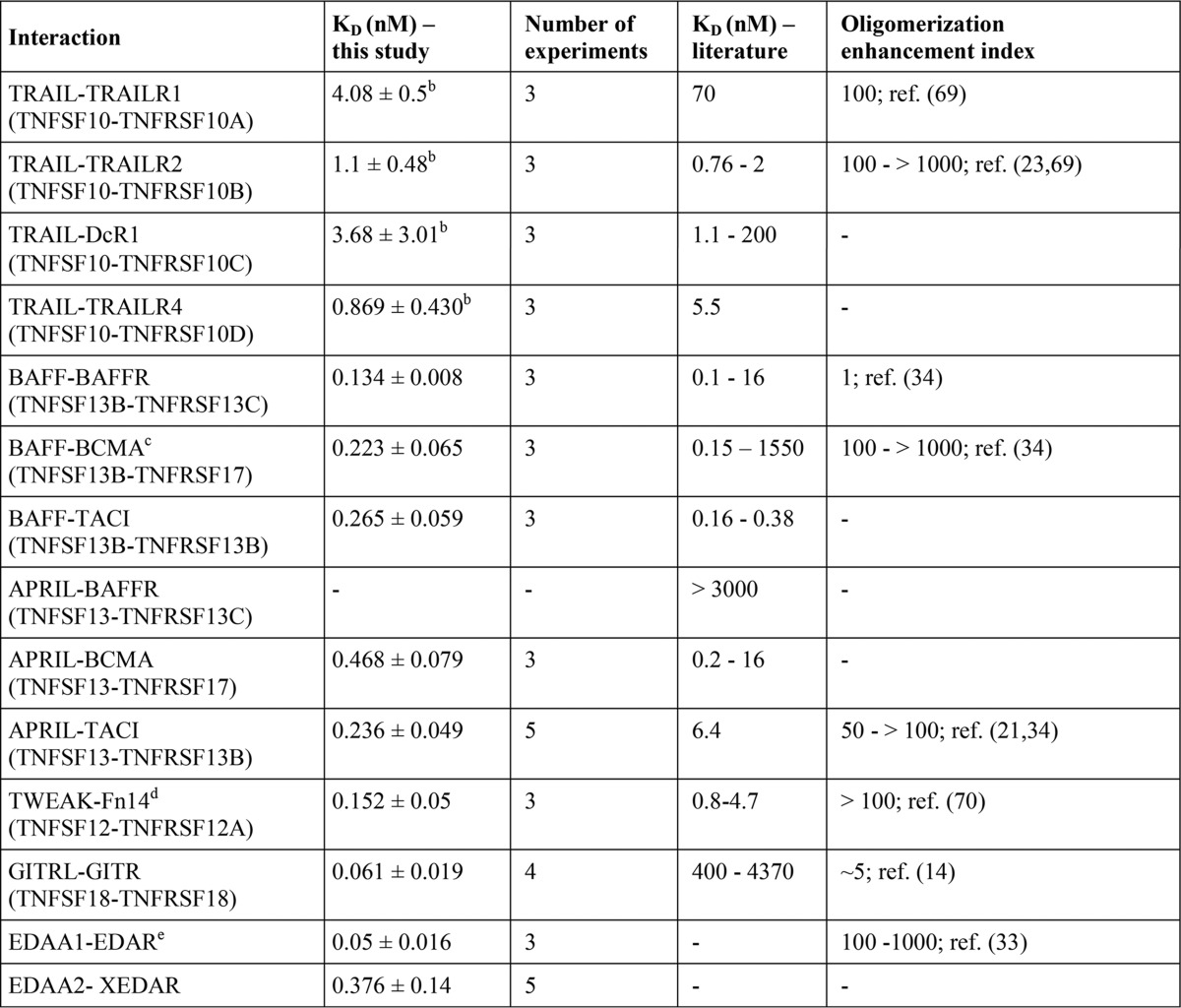
TABLE 3.
TNFSF ligand-TNFRSF receptor affinities derived from cellular binding studies at 37 ° C and their comparison with literature values
For references of affinity constants, see Table 1. All binding studies considered were performed at 37 °C and showed R2 values for non-linear regression of specific binding data of >0.95.
a In this study, binding to full-length DR3 has been determined, and in the cited study binding to a death domain deletion mutant of DR3 has been investigated.
b Our GpL-FLAG-TNC-TRAIL preparation contained significant impurities (see Fig. 1). Folding, integrity, and thus specific activity of recombinant TRAIL preparations differ notoriously, depending on the process of production and purification. The affinities of soluble GpL-TNC-FLAG-TRAIL for the various cell-expressed TRAIL receptorsindicated here could therefore be even higher.
c Functional data have been acquired with transfectants expressing an artificial BCMA-CD95 chimeric receptor.
d The enhancing effect observed in this study depends on the TWEAK-induced pathway considered. Oligomerization lowered the EC50 value for classical NF-κB signaling for 2 orders of magnitude and more but had no effect on triggering p100 processing.
e Activity data were obtained with a soluble trimeric EDA-A1 variant without the oligomerizing collagen domain of this molecule and transfectants expressing an artificial EDAR-CD95 chimeric receptor.