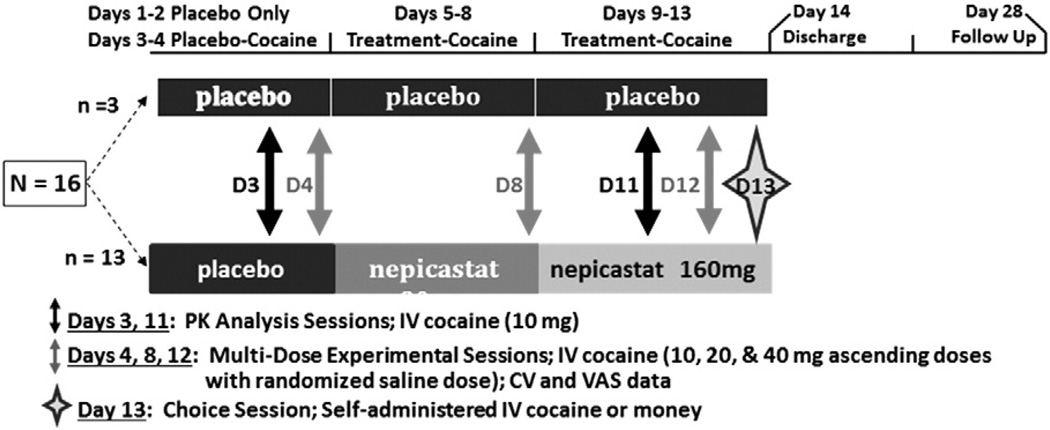
Fig. 1.
Study design. The study schema depicts the entire 28-day procedure. Subjects randomized to the placebo group (n = 3) received placebo for 13 days, while those randomized to the nepicastat group (n = 13) received placebo on Days 1–4, 80 mg of nepicastat on Days 5–8, and 160 mg of nepicastat on Days 9–13. Pharmacokinetic analyses were conducted following 10 mg of cocaine IV on Days 3 and 11. Multi-dose experimental sessions for safety and efficacy were conducted on Days 4, 8, and 12. Data were also obtained during Day 13 for the Choice Session.