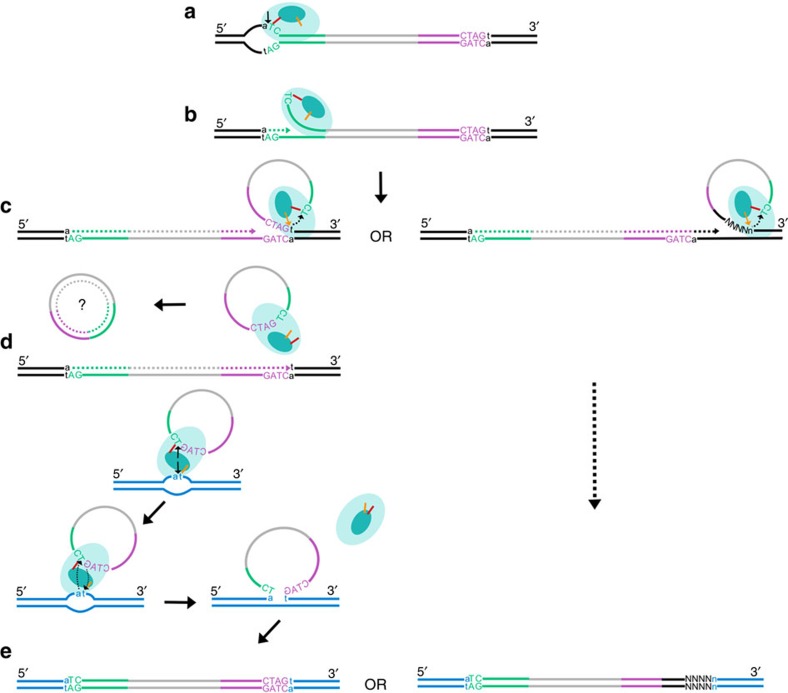
Figure 7. Proposed model of Helraiser transposition.
(a) Helraiser transposase (light blue oval) binds the LTS (green) and nicks ssDNA donor site generating a 5′-phosphotyrosine intermediate between the tyrosine residue (orange line) in the HUH nuclease active site (dark blue oval) and the transposon end. (b) A free 3′-OH group at the donor site primes DNA synthesis, while the helicase domain unwinds the dsDNA helix in a 5′ to 3′ direction. (c) The hairpin structure in the RTS (purple) induces pausing of the helicase required for the recognition and nicking of the CTAG-3′ tetrad at the RTS by the second tyrosine (yellow arrow) of the HUH domain. This generates a free 3′-OH group at the transposon RTS that attacks the first 5′-phosphotyrosine linkage generating a free ssDNA circle. The ssDNA circle is possibly converted into dsDNA circle used for further rounds of transposition. Alternatively, the transposase reads through the RTS and mobilizes host flanking sequences, thereby generating an alternative, de novo 3′-end. Further steps in transposition of the canonical transposon and the transposon containing the captured host sequence are identical. (d) Two tyrosine residues in the nuclease active site catalyse cleavage of the single-stranded target DNA and the Helitron circle, mediating the strand-transfer reaction. (e) The single-stranded transposon DNA covalently bound to the target is passively replicated and converted into the double-stranded form during the DNA synthesis phase of the cell cycle, leading to the amplification of the transposon in the host genome and transduction of host genomic sequence.