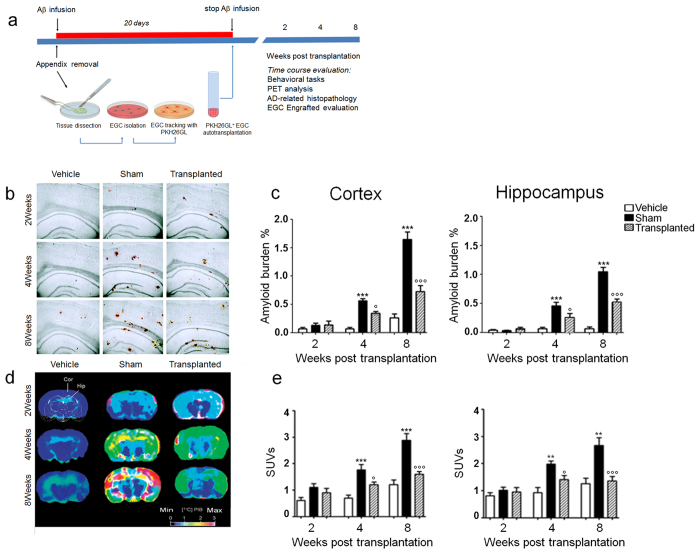
Figure 1. Autologous EGCs induced Aβ plaques degradation.
(a) Diagram showing Aβ(1–42) peptide infusion, EGCs isolation from the appendix, their transplantation in rat brain, and the time schedule for in vitro and in vivo measurements. (b) The injection of Aβ(1–42) peptide causes a time-dependent amyloid deposition in frontal cortex and hippocampus of treated rats; in EGCs transplanted rats a time-dependent reduction of Aβ plaques was instead observed (n = 10 per group; congo red staining, scale bar: 500 μm). (c) The graphs show that Aβ burden time-dependently increased in sham versus vehicle-treated rats and that EGC transplantation resulted in a significant inhibition of Aβ accumulation. In the frontal cortex (left panel) and the hippocampus (right panel) Aβ burden was significantly higher in sham (black bars) than in vehicle-treated rats (white bars) at 4 and 8 weeks (***P < 0.001), while in EGC-transplanted rats it was significantly reduced (°P < 0.05 and °°°P < 0.001 vs. sham group). (d) Representative scans of 11C-labeled Pittsburgh Compound-B positron emission tomography; Standardized uptake values (SUV) images of Aβ binding within the region of interest (ROIs) in the frontal cortex (COR) and hippocampus (HIP) of rats (n = 6 per group). (e) Relative SUVs for selected ROIs in the cortex (left panel) and hippocampus (right panel). Data were expressed as mean ± SEM; **P < 0.01, ***P < 0.001 vs. vehicle; °P < 0.05; °°° P < 0.001 vs. sham; two-way ANOVA.