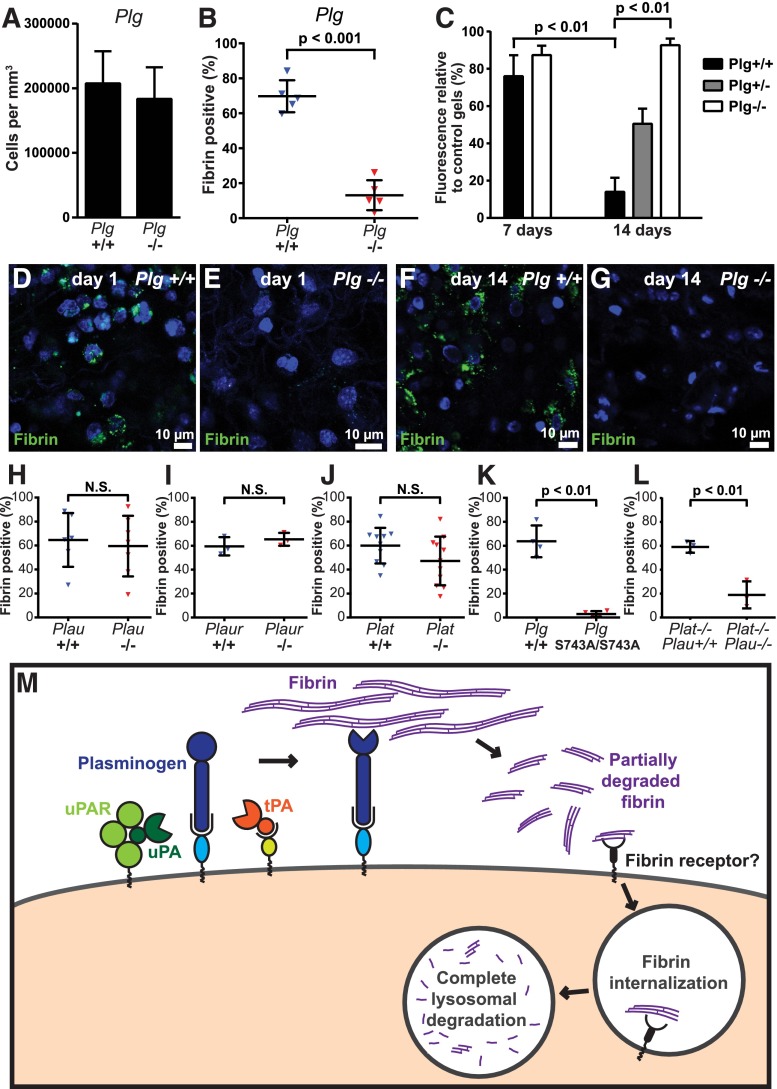
Figure 7.
Plasminogen and plasmin activity is critical for endocytic fibrin degradation. (A) Loss of plasminogen does not affect cell recruitment to fibrin implantation zones. Total cell numbers in implantation zones from wild-type mice (left bar, N = 5 mice) and Plg−/− littermates (right bar, N = 5 mice). Data are shown as mean values with standard deviations. (B-G) Reduced fibrin endocytosis in plasminogen-deficient mice. (B) Enumeration of the fibrin endocytosing fraction of cells in implantation zones from wild-type mice (blue triangles) and Plg−/− littermates (red triangles). Data are shown as individual values for each mouse, as well as mean values and standard deviations. Significance was determined by Student t test (2 tailed). (C) Loss of plasminogen prevents extravascular fibrin degradation. Fluorescent fibrin gels were placed in subcutaneous space of Plg+/+ (black bars, N = 3), Plg+/− (gray bar, N = 4), and Plg−/− (white bars, N = 3 [day 7] and N = 6 [day 14]) mice. The gels were extracted at 7 and 14 days after implantation, and fluorescence was measured. Data are expressed as percent residual fluorescence compared with control gels stored for the identical time period in the dark at 37°C in PBS with sodium azide. Significance was determined by 2-way ANOVA (comparison of days) and 1-way ANOVA (comparison of genotypes). (D-G) Representative examples of implantation zone from wild-type (Plg+/+) (D,F) and littermate plasminogen-deficient (Plg−/−) (E,G) mice 24 hours (D-E) and 14 days (F-G) after fibrin implantation. Multiple cells with endocytosed fibrin (green) are found in wild-type mice, but not in plasminogen-deficient mice, at both time points. (H-J) Individual deficiencies in uPA, uPAR, and tPA do not diminish endocytic fibrin uptake. Enumeration of fraction of cells in implantation zones from wild-type mice (blue triangles) and Plau−/− littermates (red triangles) (F), wild-type mice (blue triangles) and Plaur−/− littermates (red triangles) (G), and wild-type mice (blue triangles) and Plat−/− littermates (red triangles) (H). Data are shown as individual values for each mouse, as well as mean values and standard deviations. Significance was determined by Student t test (2 tailed). (K) Plasmin enzymatic activity is required for fibrin endocytosis. Enumeration of fraction of cells in implantation zones from wild-type mice (blue triangles) and PlgS743A/S743A littermates (red triangles). Data are shown as individual values for each mouse, as well as mean values and standard deviations. Significance was determined by Student t test (2 tailed). (L) Functional overlap of uPA and tPA in mediating fibrin endocytosis. Enumeration of fraction of cells in implantation zones from Plat−/− mice (blue triangles) and Plat−/−;Plau−/− littermates (red triangles). Data are shown as individual values for each mouse, as well as mean values and standard deviations. Significance was determined by Student t test (2 tailed). (M) Hypothesized mechanism of extravascular fibrin clearance by inflammatory CCR2 macrophages. Cell-surface receptor-bound plasminogen on macrophages recruited to fibrin deposits is activated by cell-surface receptor-bound uPA and tPA. The resulting cell-surface–bound plasmin, protected from inactivation by α2-antiplasmin, partially degrades fibrin. The partially degraded fibrin is endocytosed by the CCR2-macrophages, possibly through an unidentified fibrin receptor different from αMβ2 and ICAM-1. Additional partially degraded fibrin may be made available for endocytosis by non–cell-surface–dependent plasminogen activation pathways (not shown). Fibrin-containing endocytic vesicles deliver their cargo to lysosomes, resulting in complete degradation of fibrin by lysomal proteases. N.S., not significant.