Abstract
This work characterized airborne particles that were generated from the weighing of bulk, multi-wall carbon nanotubes (CNTs) and the manual sanding of epoxy test samples reinforced with CNTs. It also evaluated the effectiveness of three local exhaust ventilation (LEV) conditions (no LEV, custom fume hood, and biosafety cabinet) for control of particles generated during sanding of CNT-epoxy nanocomposites. Particle number and respirable mass concentrations were measured using an optical particle counter (OPC) and a condensation particle counter (CPC), and particle morphology was assessed by transmission electron microscopy. The ratios of the geometric mean (GM) concentrations measured during the process to that measured in the background (P/B ratios) were used as indices of the impact of the process and the LEVs on observed concentrations. Processing CNT-epoxy nanocomposites materials released respirable size airborne particles (P/B ratio: weighing = 1.79; sanding = 5.90) but generally no nanoparticles (P/B ratiô1). The particles generated during sanding were predominately micron-sized with protruding CNTs and very different from bulk CNTs that tended to remain in large (>1 μm) tangled clusters. Respirable mass concentrations in the operator’s breathing zone were lower when sanding was performed in the biological safety cabinet (GM = 0.20 μg/m3) compared to those with no LEV (GM = 2.68 μg/m3) or those when sanding was performed inside the fume hood (GM = 21.4 μg/m3; p-value < 0.0001). The poor performance of the custom fume hood used in this study may have been exacerbated by its lack of a front sash and rear baffles and its low face velocity (0.39 m/sec).
Keywords: Multi-wall carbon nanotubes, CNT, nanocomposite, respirable mass concentration, exposure assessment
INTRODUCTION
Carbon nanotubes (CNTs) can be incorporated into polymeric materials to form nanocomposite materials.(1) Compared to the polymeric material alone, these nanocomposites have increased resistance to strain and tensile strength, improved electrical and thermal properties, and enhanced ability to bridge cracks.(1) Products that incorporate CNT nanocomposites include sporting goods, automotive parts, and electronics,(2) and the number of industrial and commercial applications of these nanocomposites are projected to increase as their production cost decreases.(2,3)
Workplace exposure to airborne CNTs may represent an occupational hazard.(4) Toxicological studies have associated exposure to CNTs with adverse pulmonary (granulomas, fibrosis, diminished resistance to pathogenic attacks),(5-9) cardiac (oxidative damage, atherosclerotic lesions)(10) and dermal health effects (oxidative stress, increased cell apoptosis and necrosis).(9,11)
The production of CNT nanocomposites involves the processing of bulk CNTs, which are CNTs not incorporated in a matrix, and CNTs embedded in a polymer matrix such as epoxy resin.(3) Weighing or pouring bulk CNTs has been observed to release only small quantities (<53μg/m3) of airborne particles in laboratory(12,13) and field conditions.(13) Appreciable respirable concentrations have been observed only after vigorous agitation.(13) In contrast, the mechanical processing of CNT nanocomposites may impart greater energy to produce appreciable quantities of airborne particles during typical workplace operations. Kohler et al.(14) suggest that the way CNTs are incorporated in the matrix and the mechanism by which a nanocomposite degrades determine the likelihood and form of their release. Mechanical processing of nanocomposites may release micron-sized aggregate polymer-CNT particles or CNTs in a dispersive nanoparticulate state.(3,14)
Laboratory enclosures have been designed to protect workers from exposure during handling and processing of hazardous substances. The effectiveness of fume hoods as a control measure during nanoparticle manipulation has been investigated by Tsai et al.(15) They measured airborne concentration while dry nanopowders were handled under three fume hoods and a range of operating conditions (variable sash height and face velocity). Their results showed that the release of airborne nanoparticles from within a fume hood into the laboratory environment is highly dependent upon hood design and hood conditions (sash height, face velocity). Further, they suggested that more sophisticated hood designs, such as air-curtain biosafety cabinets, may be more effective in containing nanoparticles.(15) The effectiveness of enclosures to contain particulate release, however, has not been investigated during the manipulation of nanocomposite materials containing CNTs.
The goals of this study were 1) to characterize airborne particles during handling of bulk CNTs and the mechanical processing of CNT nanocomposites and 2) to evaluate the effectiveness of local exhaust ventilation (LEV) hoods to capture airborne particles generated by sanding CNT nanocomposites.
METHODS
Manufacturing Process
This study was conducted in a facility that produces test samples composed of epoxy reinforced with CNTs. The test samples are rectangular sticks of CNT-epoxy with dimensions of 12.5 × 1.3 × 0.5 cm that are used to evaluate the effect of formulation variables on the properties of the nanocomposites. Specific details of this process are not reported at the request of the manufacturer, only the details required to interpret results from an occupational health and safety perspective are presented.
To produce the test samples, bulk multi-wall CNTs with 10-50 nm outer diameter and 1-20 μm length (Baytubes, Bayer Material Science LLC, Pittsburg, PA) are weighed and mixed with epoxy. This mixture is poured into a mold designed to produce four test samples at a time and baked in an oven for several hours. Then the hardened nanocomposite test samples are broken apart manually, and each one is manually sanded to remove excess material until final dimensions are achieved.
Airborne Particle Measurement and Characterization
Airborne particle number and respirable mass concentrations were measured with two direct-read instruments: a condensation particle counter (CPC), and an optical particle counter (OPC). The CPC (model 3007, TSI Inc., St. Paul, MN) was used to provide total particle number concentration for particles that ranged in diameter from 0.01 to 1 μm. The OPC (Portable Dust Monitor series 1.108, GRIMM Technologies, Douglasville, GA) was used to provide particle number concentration in 15 size channels from 0.3 to 20 μm.
Respirable mass concentrations were estimated from the OPC data using the respirable particulate matter (RPM) fraction defined by ACGIH.(16) This fraction consists of those particles that were captured as specified by the following collection efficiency:
| (1) |
where dOPC,i is the midpoint diameter of the OPC ith channel in μm, and F(x) is the cumulative probability function of the standardized normal variable x,
For each OPC measurement, respirable mass concentration, MR, was calculated as:
| (2) |
where NOPC,i is the number concentration indicated by the OPC for a given size channel i, and ρ is the particle density (2.25 g/cm3 for epoxy resin).
The morphology of representative airborne particles was observed by transmission electron microscopy (TEM). Samples for microscopy were collected with a sampler similar to that described by Tsai et al.(15) It consisted of a copper TEM grid (300 mesh with carbon type-b film, 01813-F, Ted Pella, Inc., Redding, CA) affixed with carbon tape onto the center of the face of a polycarbonate membrane filter (E0055-MB, SPI Supplies, West Chester, PA). This filter was then mounted in an open-face conductive filter cassette (25 mm, 225-3-23, SKC Inc., Eighty Four, PA). Airflow was pulled through the sampler with a personal sampling pump (Buck Basic-5, A.P. Buck Inc., Orlando, FL) at 1 L/min. The copper grids were analyzed under TEM (JEOL JEM-1230, Peabody, MA) to characterize the size and morphology of a representative subset of the collected airborne particles.
Process Measurements
Airborne concentrations were measured during two processes: weighing bulk CNTs and sanding epoxy nanocomposite test sticks. To simulate weighing, 600 mg of the bulk CNTs were transferred by scooping material between two 50 mL beakers for three five-minute intervals at a rate of ~ 1 scoop/sec. Each scoop contained approximately 50 mg of CNT material. These tests were conducted in a filtered glove box with the CPC and OPC located inside the glove box with their inlets positioned 7.5 cm away from and oriented towards the weighing process. Background concentrations were measured for 15 min inside the glove box before the process began. One TEM sample was collected inside the glove box during the entire weighing process. Additional TEM samples were prepared by artificially depositing CNTs on TEM grids.
To study the sanding process, an operator manually sanded epoxy test sticks that contained 2% by weight CNTs with sandpaper (220 grit, model 20240, 3M, St Paul, MN). The operator wore a full-face respirator with particulate filters (Full Facepiece 6700, 3M, St Paul, MN). Aerosol concentrations were measured for 15-30 min in two locations (adjacent to the sanding process and in the operator’s breathing zone). For process measurements (termed ‘inside enclosure’ measurments),the inlets of the CPC and OPC were positioned 7.5 cm away from and oriented towards the sanding process. For breathing zone measurements, two electrically conductive, flexible tubes (1.4 m long with an inner diameter of 0.48 cm) were used to transport the aerosol from just outside of the respirator to a second CPC and OPC. TEM samples were collected at 7.5 cm from the sanding surfaces. Diffusion losses introduced by the presence of the sampling tube on CPC measurements were estimated from theory to be 23 percent for 10 nm particles and less than 5 percent for particles larger than 40 nm.(17) The bias introduced by the sampling tube on respirable mass concentration was estimated to be less than −5 percent.(18)
For sanding, source and breathing zone measurements were taken under three LEV conditions (no LEV, a custom fume hood, and a biological safety cabinet). With no LEV, the sanding process was conducted on a 1.2 m by 2.2 m work table. The custom fume hood consisted of a simple vented enclosure that allowed airflow along all sides of the back panel. The custom fume hood had no front sash or rear baffles, and its dimensions were 0.57 m (height) by 1.33 m (width) by 0.76 m (depth). The face velocity of the hood was measured at the center of 21 equally spaced positions according to the procedures outlined by ANSI/ASHRAE 110-1995.(19) The biological safety cabinet was class II type A2 (Sterilgard III 303, Baker Co., Sanford, ME) with dimensions of 0.53 m (height) by 0.7 m (width) by 0.45 m (depth). The cabinet was tested for performance evaluation and operated with a 20 cm (8 inch) sash height. During all tests, the LEV contained no additional equipment that may have blocked airflow, and no other equipment external to the LEV was operated in the room that may have generated airborne particles. Background measurements were taken for 30 min prior to each test in the rooms as well as inside the LEV enclosures in the same location as the process measurements.
Data Analysis
All particle number and respirable mass concentrations were tested for normality using Shapiro-Wilk test before and after performing a log transformation. The geometric mean (GM) and geometric standard deviation (GSD) were obtained for all number and respirable mass concentrations at each condition tested. Process-to-background ratios (P/B ratios) were calculated for all measurements with the formula
| (3) |
Breathing zone and process GM concentrations were matched with their respective background measurements.
Analysis of variance (ANOVA) was used to compare respirable mass concentrations measured in the operator’s breathing zone during the sanding process with and without control measures. Not all data passed the Shapiro-Wilk test for normality; therefore the concentrations were further compared by Kruskal-Wallis nonparametric test. The Tukey-Kramer Honestly Significant Difference (HSD) test was used to compare GM concentrations observed for different LEV conditions.
RESULTS
The GM and GSD of the observed particle number and respirable mass concentrations are summarized by process in Table I. Table II presents GM and GSD of the particle number and respirable mass concentrations obtained under various LEV conditions during the sanding process. The P/B ratios indicate the relative impact of the process (weighing or sanding) or of the LEV on observed concentrations. Values near unity indicate that the process generated little aerosol, whereas values progressively greater than unity indicate that the process increased aerosol concentrations.
TABLE I.
Particle number and respirable mass concentrations observed during the weighing and sanding processes. N represents the number of data points logged by the instruments during one run.
| a. Number Concentration | ||||
|---|---|---|---|---|
|
| ||||
| Process | N |
GM
#/cc |
GSD | P/B Ratio |
| Weighing | 300 | 166 | 1.08 | 1.06 |
| Sanding | 100 | 3889 | 1.48 | 1.04 |
|
| ||||
| b. Respirable Mass Concentration | ||||
|
| ||||
| Process | N |
GM
μg/m3 |
GSD | P/B Ratio |
|
| ||||
| Weighing | 51 | 0.03 | 3.50 | 1.79 |
| Sanding | 130 | 2.68 | 6.57 | 5.90 |
TABLE II.
Particle number and respirable mass concentrations during sanding with and without local exhaust ventilation (LEV). N represents the number of data points logged by the instruments during one run.
| a. Number Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Breathing Zone
|
Inside Enclosure
|
|||||||
| LEV | N |
GM
#/cc |
GSD | P/B Ratio | N |
GM
#/cc |
GSD | P/B Ratio |
|
Custom
Hood |
210 | 1989 | 1.07 | 1.03 | 211 | 1742 | 1.05 | 1.01 |
| None | 100 | 3889 | 1.48 | 1.04 | 119* | 3765* | 1.07* | 1.01* |
|
Biosafety
Cabinet |
101 | 1350 | 1.07 | 1.05 | 108 | 0.06 | 2.41 | 2.35 |
|
| ||||||||
|
b. Respirable Mass Concentration
| ||||||||
|
Breathing Zone
|
Inside Enclosure
|
|||||||
| LEV | N |
GM
μg/m3 |
GSD | P/B Ratio | N |
GM
μg/m3 |
GSD | P/B Ratio |
|
| ||||||||
|
Custom
Hood |
190 | 21.4 | 5.85 | 24.4 | 93 | 31.5 | 12.1 | 28.6 |
| None | 130 | 2.68 | 6.57 | 5.90 | 80* | 10.6* | 7.02* | 23.2* |
|
Biosafety
Cabinet |
101 | 0.20 | 2.12 | 0.66 | 108 | 0.03 | 39.7 | 3.47 |
For LEV=none (work table), “inside enclosure” measurements were taken adjacent to the source in the same relative location as those taken inside the custom fume hood and the biosafety cabinet.
Influence of Process on Airborne Concentrations
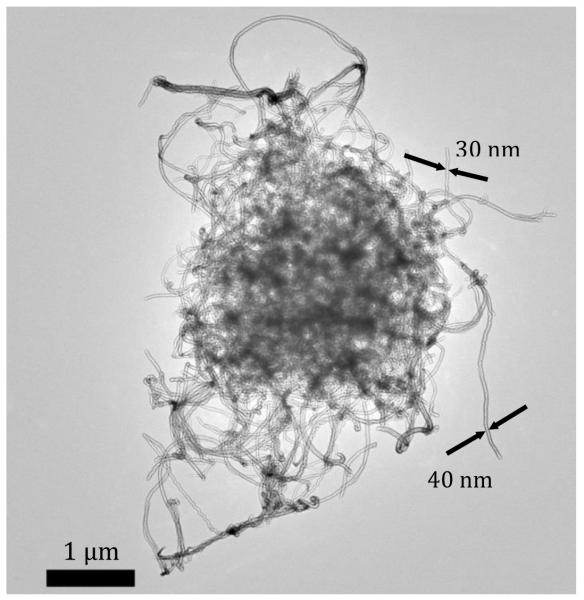
The weighing process contributed little to observed particle number concentrations (Table I-a; P/B ratio = 1.06). It did influence mass concentration (Table I-b; P/B ratio = 1.79), although very low computed respirable mass concentrations were observed inside the glove box during weighing (GM = 0.03 μg/m3) and in background measurements (GM = 0.02 μg/m3). Electron microscope analysis revealed no CNT particles deposited on the TEM grids during the weighing process. The CNTs artificially deposited onto TEM grids appeared as large bundles (>1 μm) containing many tangled nanotubes (Figure 1).
FIGURE 1.
Bulk 10-50 nm outer diameter multi-wall CNTs with many tangled nanotubes.
During the sanding process, nanoparticle number concentrations were negligible compared to background concentrations (Table I-a; P/B ratio = 1.04), indicating that nanoparticles did not disperse to any great extent. Respirable mass concentrations, however, were elevated in the breathing zone (Table I-b; P/B ratio = 5.90).
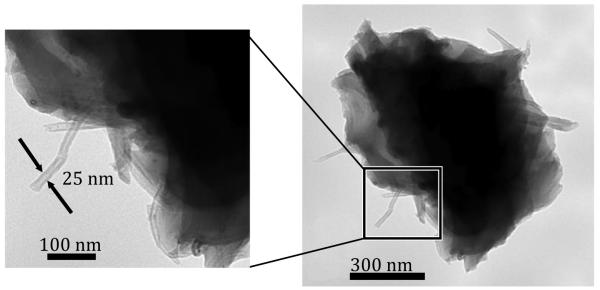
The particles collected during sanding were predominantly large (>300 nm) and irregular in shape (a representative particle is shown in Figure 2). These particles commonly had protuberances that emerged from the perimeter of the particles. These protuberances had an outer diameter between 10 and 50 nm, which is consistent with that of the CNTs.
FIGURE 2.
Sanding particle with detail of protruding fibers (TEM image).
Influence of Local Exhaust Ventilation During Sanding
Particle number concentrations measured in the breathing zone during sanding were negligible compared to background for all three LEV conditions (Table II-a; breathing zone P/B ratios: custom fume hood = 1.03, no LEV = 1.04, biosafety cabinet = 1.05). The P/B ratios were dissimilar for process measurements made inside enclosures (Table II-a; inside enclosure). Inside the fume hood the P/B ratio was 1.01, very close to unity. Inside the biosafety cabinet, the P/B ratio was 2.35, indicating a 235% increase in number concentrations; however, the actual concentrations in this condition were very close to zero (GM = 0.06 particles/cm3), with a maximum of 8 particles/cm3.
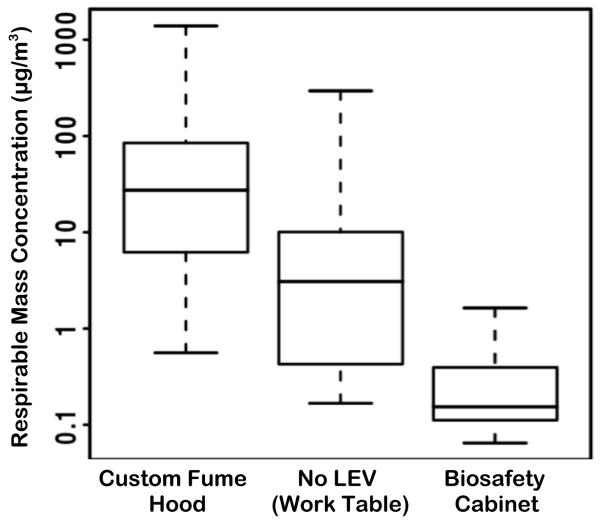
Respirable mass concentrations in the worker’s breathing zone varied substantially by LEV (Table II-b; breathing zone). Figure 3 presents box-and-whisker plots of these breathing zone measurements. The ANOVA test reported significant differences between the three LEV conditions (p-value <0.0001 confirmed by Kruskal-Wallis nonparametric test), and the Tukey-Kramer HSD test confirmed that all pair-wise comparisons were significantly different (p-values <0.00001). Inside the LEV enclosures, respirable mass concentrations were considerably higher than background levels. The P/B ratio was 28.6 for the fume hood LEV and 3.47 for the biosafety cabinet LEV (Table II-b; inside enclosure).
FIGURE 3.
Box-and-whisker plot of log-transformed respirable mass concentrations. Box parameters represent median, lower and upper quartiles. Whiskers represent sample min and max.
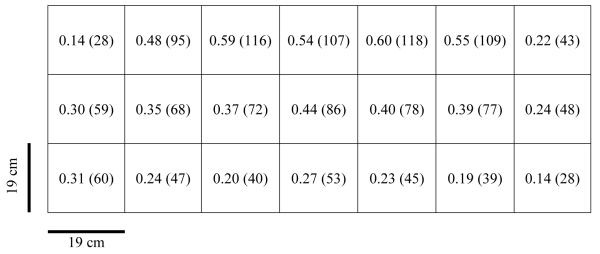
The average face velocity of the fume hood, measured with a thermal anemometer (VelociCalc 8360, TSI, Inc. St. Paul, MN), was 0.39 m/sec (76 ft/min). As shown in Figure 4, the air velocity near the lower part of the hood’s face, closer to the sanding surface, was on average 0.23 m/sec (45 ft/min) while in the upper area it averaged 0.45 m/sec (88 ft/min), with maximum flow at 0.60 m/sec (118 ft/min) in the upper central area of the hood’s face.
FIGURE 4.
Air velocities in m/sec (ft/min) measured at the face of the custom fume hood.
DISCUSSION
Processing CNT-nanocomposite materials releases respirable size airborne particles but generally no nanoparticles. The finding that P/B ratios for number concentration were near unity (Table I-a) indicates that nanoparticles did not disperse to any great extent either when weighing bulk CNTs or during the sanding process. This result is consistent with the work of Baron et al.(12) and Maynard et al.(13) who found that CNTs are difficult to separate into isolated particles and that the particles released during handling of bulk CNTs tend to be larger than 1 μm. Baron et al.(12) also found that aerosol generation rates were typically two orders of magnitude lower when comparing CNTs to fume alumina bulk materials (a material formed from nanometer-sized primary particles with similar low bulk density to CNTs).
Respirable mass concentrations in the breathing zone of the operator were elevated compared to background during sanding without LEV (P/B ratio = 5.90). However, the geometric mean (GM = 2.68 μg/m3) was considerably lower than the only applicable occupational standard, the recommended American Conference of Governmental Industrial Hygienists (ACGIH) respirable particles threshold limit value for particles not otherwise specified (PNOS TLV – 3000 μg/m3).(16)
The P/B ratio for respirable mass concentration of 1.79 measured during the weighing process (Table I-b) appears to indicate that the process did influence mass concentrations. However, very low respirable mass concentrations were observed during weighing (0.03 μg/m3), and when concentrations are extremely low, even small fluctuations will be reflected as a substantial increase in the P/B ratios. Such small fluctuations may be related to normal background variability (Background GM 0.02 μg/m3, GSD 2.06) rather than actual increases in respirable mass concentrations while weighing CNTs.
The morphology of particles generated during sanding CNT-epoxy nanocomposites was very different from the bulk CNTs (Figures 1 and 2). The particles collected in the operator’s breathing zone during sanding were micron-sized with protruding features (Figure 2). Their morphology suggests that they are epoxy particles and that the protrusions are embedded CNTs which extend outward beyond the perimeter of the particle. No CNTs were observed free from composite material for the manual sanding studied in this work. It is, however, possible that free CNTs are generated and would be observed at higher concentrations or when mechanical sanding is performed.
The fact that free CNTs were not liberated from the epoxy is consistent with research on asbestos containing materials. Asbestos fibers that are encapsulated or bonded with other materials, such as resins, have been found to have a limited potential for airborne release.(20) Aggressive manipulation, such as sanding, sawing, or drilling of polymer matrices containing asbestos fibers has been found to produce airborne concentrations of asbestos that are fifty to a hundred fold less than the historical standards set by OSHA and ACGIH for worker protection, and at least threefold less than the current 8-hour TWA occupational exposure limits.(20)
No particles were found on the TEM samples collected during the raw CNT weighing simulation of this study. The CNTs that were artificially deposited onto TEM grids appeared mainly as large (>1 μm), tangled clusters. Similar large clusters were observed to deposit on surface inside the glove box during the process simulation. In contrast, exposure to asbestos fibers has been found to occur when workers handle and process raw asbestos fibers or friable products such as insulation.(20)
Toxicological studies have focused to date on the adverse health effects associated with exposure to bulk CNTs. Our findings suggest that inhalation exposure to bulk CNTs is likely to be low in activities such as weighing. In contrast, sanding does generate respirable particles that can enter a worker’s breathing zone, and these particles were very different morphologically than bulk CNTs. Given the lack of toxicity data for this type of particles, it cannot be determined whether exposure to the sanding particles generated at the concentrations measured in this study presents a risk to workers’ health. Precautions such as the use of personal protective equipment or working within a hood that meets design specifications and standards or a biological safety cabinet should be taken until health effects are better understood.
LEV conditions had a substantial influence on respirable mass concentrations in the operator’s breathing zone. Breathing zone concentrations were elevated when sanding took place inside the custom fume hood (Table II-b and Figure 3). The facemask of the operator performing the sanding task in the custom hood was consistently covered with particles that had to be removed with the use of dry wipes in order to improve visibility.
The poor performance of the custom hood used in the current study may have been exacerbated by its lack of a front sash, its lack of rear baffles to distribute the airflow,(21) and its low face velocity. The ACGIH recommends an inward face velocity for a fume hood of 0.4-0.5 m/sec (80-100 ft/min) with a velocity difference at any point in the face opening no greater than 10% of the average velocity.(22) The maximum air velocity of the custom fume hood used in this study (0.60 m/sec; 118 ft/min) was 200% greater than the average velocity 0.23 m/sec (45 ft/min). The uneven velocity distribution may have affected airflow patterns around the worker and the ability of the custom fume hood to remove particles from the operator’s breathing zone. Operator movements may increase air turbulence inside a hood, compromising its effectiveness to remove contaminants and pulling dust particles towards the operator.(21) Similar results were found by Tsai et al.,(15) who observed the transport of alumina nanoparticles from within a fume hood that met ACGIH criteria for face velocity into the breathing zone of the operator. However, Tsai et al.(15) also identified that containment in a conventional fume hood is highly dependent upon its design and operating conditions. These findings corroborate the importance of effective hood design details such as the use of air currents and how eddy currents may influence hood containment efficiency.
In contrast, the breathing zone respirable mass concentrations (Table II-b and Figure 3) were significantly lower when sanding was conducted in the biosafety cabinet compared to the other LEV conditions. Airflows are substantially different in biosafety cabinets, mainly because of the presence of a front suction slot, which creates an air curtain across the front aperture.(23,24) This air curtain separates the outside atmosphere and the inside space of the cabinet, preventing escape of contaminants from containment. These observations suggest that biosafety cabinets may be an appropriate control measure to contain exposure during sanding of CNT-reinforced epoxy
The P/B ratio less than unity observed during sanding with the biosafety cabinet LEV (Table II-b; 0.66) suggests that the mass concentration was greater in the background than that during sanding. This observation was likely due to fluctuations in background concentrations and to the extremely low mass concentrations (GM = 0.20 μg/m3). Small fluctuations in background concentrations inside the biosafety cabinet could have substantially affected the P/B ratio, but do not appear to be related to the sanding task. An analogous situation applies to the near zero particle number concentrations inside the biosafety cabinet (Table II-a; GM = 0.06 particles/cm3) and its associated high P/B ratio (2.35).
There are several limitations of this work. The fact that this work was conducted in an industrial facility under ‘as is’ conditions limited our ability to control experimental variables and perform extensive repeated measurements. Exposures during sanding may be highly variable depending on how forcefully the sanding is performed and whether it is done continuously over a 30-minute period. Future studies should investigate how process details affect particulate generation and establish how the amount of material dispersed from the sandpaper contributes to aerosol measurements. Although aerosol concentrations for all three LEV conditions were continuously logged over a 15-30 minute period, the statistical parameters may not be representative of what is expected when a task is repeated several times. Lastly, it may be difficult to generalize the findings of this work to nanocomposite materials containing non-fibrous nanoparticles since non-fibrous particles may be more easily aerosolized than fibrous particles.
CONCLUSIONS
This study demonstrated that weighing bulk CNTs and sanding epoxy containing CNTs generates few airborne particles that are nano-sized. Furthermore it was demonstrated that sanding epoxy containing CNTs may generate micron-sized particles with CNTs protruding from the main particle core. These epoxy particles with embedded CNTs, and not the bulk CNTs, appear to represent the relevant worker’s exposure during the production process examined in this study. The toxicity of epoxy particles containing CNTs is unknown and should be the topic of future studies. Without such knowledge, precautions, such as the use of LEV, should be applied to avoid exposures resulting from manipulating epoxy that contains CNTs. This study also identified that a biological safety cabinet was more effective than a custom fume hood to control airborne exposures resulting from sanding epoxy containing CNTs. Future studies should characterize exposures that occur throughout the various steps involved in the manufacturing of products that contain nanomaterials.
REFERENCES
- 1.Lau AK, Hui D. The revolutionary creation of new advanced materials—carbon nanotube composites. Composites Part B: Engineering. 2002;33(4):263–277. [Google Scholar]
- 2.Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008;17:438–447. doi: 10.1007/s10646-008-0210-4. [DOI] [PubMed] [Google Scholar]
- 3.Lam C, James JT, McCluskey R, Arepalli S, Hunter RL. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Critical Reviews in Toxicology. 2006;36(3):189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 4.Tejral G, Panyala NR, Havel J. Carbon nanotubes: Toxicological Impact on Human Health and Environment. J. Appl. Biomed. 2009;7:1–13. [Google Scholar]
- 5.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days After Intratracheal Instillation. Toxicological Sciences. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 6.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR. Comparative Pulmonary Toxicity Assessment of Single-wall Carbon Nanotubes in Rats. Toxicological Sciences. 2003;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 7.Muller J, Huaux F, Moreau N, Misson P, Jean-Francois H, Delos M, Arrasa M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Shvedova AA, Kisin ER, Mercer R, Murray, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Bon-Ki Ku, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289(5):L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 9.Shvedova AA, Castranova V, Kisin D, Schwagler-Berry AR, Murray VZ, Gandelsman A, Maynard P. Baron: Exposure to Carbone Nanotube Material: Assessment of Nanotube Cytotoxicity Using Human Keratocyte Cells. Journal of Toxicology and Environmental Health. 2003;66(Part A):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Huldermen T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova AA, Luster MI, Simeonova PP. Cardiovascular Effects of Pulmonary Exposure to Single-Wall Carbon Nanotubes. Environmental Health Perspectives. 2007;115(3):377–382. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol. Lett. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Baron PA, Maynard AD, Foley M. Evaluation of aerosol release during the handling of unrefined single walled carbon nanotube material. NIOSH DART-02-191, National Institute of Occupational Safety and Health, Cincinnati, OH, NTIS PB2003–102401. 2003:1–22. [Google Scholar]
- 13.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single walled carbon nanotube material. J. Toxicol. Environ. Health. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 14.Köhler AR, Som C, Helland A, Gottschalk F. Studying the Potential Release of Carbon Nanotubes Throughout the Application Life Cycle. Journal of Cleaner Production. 2008;16:927–937. [Google Scholar]
- 15.Tsai SJ, Ada E, Isaacs JA, Ellenbecker MJ. Airborne nanoparticle exposures associated with the manual handling of nanoalumina and nanosilver in fume hoods. J. Nanopart. Res. 2009;11:147–161. [Google Scholar]
- 16.American Conference of Governmental Industrial Hygienists . TLVs and BEIs. ACGIH; Cincinnaty, OH: 2009. [Google Scholar]
- 17.Brockmann JE. Sampling and Transport of Aerosol. In: Baron PA, Willeke K, editors. Aerosol Measurement: Principles, Techniques, and Applications. Second Edition Wiley-Interscience; New York: 2001. pp. 143–195. [Google Scholar]
- 18.Peters TM, Volkwein JC. Analysis of Sampling Line Bias on Respirable Mass Measurement. Applied Occupational and Environmental Hygiene. 2003;18(6):458–465. doi: 10.1080/10473220301418. [DOI] [PubMed] [Google Scholar]
- 19.American National Standards Institute (ANSI) American Society of Heating Refrigeration and Air Conditioning Engineers (ASHRAE) Method of testing performance of laboratory fume hoods (ANSI/ASHRAE Standard 110–1995) ASHRAE; Atlanta, GE: 1995. [Google Scholar]
- 20.Mowat F, Bono M, Lee RJ, Tamburello S, Paustenbach D. Occupational Exposure to Airborne Asbestos from Phenolic Molding Material (Bakelite) During Sanding, Drilling, and Related Activities. Journal of Occupational and Environmental Hygiene. 2005;2:497–507. doi: 10.1080/15459620500274237. [DOI] [PubMed] [Google Scholar]
- 21.Wunder J. Quantitative Fume hood Evaluation for Operator Safety. Chemical Health and Safety. 2000;7(4):26–30. [Google Scholar]
- 22.ACGIH . Industrial Ventilation - a Manual of Recommended Practices. 26th Ed ACGIH; Cincinnati, OH: 2007. [Google Scholar]
- 23.Huang RF, Chou CI. Flow and Performance of an Air-curtain Biological Safety Cabinet. Ann. Occup. Hyg. 2009;53(4):425–440. doi: 10.1093/annhyg/mep020. [DOI] [PubMed] [Google Scholar]
- 24.Kruse RH, Puckett WH, Richardson JH. Biological Safety Cabinetry. Clinical Microbiology Reviews. 1991;4(2):207–241. doi: 10.1128/cmr.4.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]