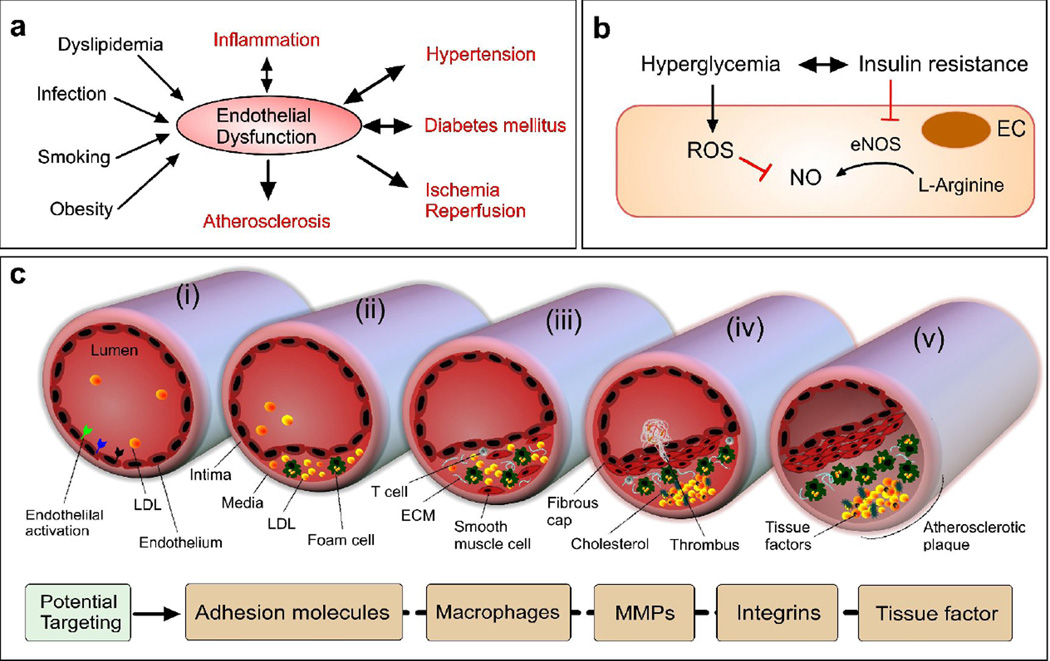
Figure 3. Endothelial disorder in metabolic and cardiovascular diseases.
(a) Key EnD inducers and EnD-associated diseases. (b) A key EnD mechanism in diabetes. NO is formed from L-arginine by eNOS. In diabetes characterized by insulin resistance and hyperglycemia, EnD results from reduced production of NO. This arises through decreased activation of eNOS due to insulin resistance and increased breakdown of NO by ROS, promoted by hyperglycemia. (c) Initiation and progression of atherosclerosis with an activated endothelium (adapted from [95]). Atherogenic lipoproteins enter the intima and aggregate within the extracellular intimal space (i). Unregulated uptake of these atherogenic lipoproteins by macrophages leads to the generation of foam cells (ii). In addition to monocytes, other types of leukocyte, particularly T cells, are recruited to atherosclerotic lesions and cause chronic inflammation. The growth of plaque induces tissue remodeling (iii). The foam cells release cellular debris and crystalline cholesterol. Smooth muscle cells form a fibrous cap beneath the endothelium, contributing to the formation of a necrotic core within the plaque. The resulting non-obstructive plaque may rupture, resulting in the formation of a thrombus in the lumen (iv), which can lead to tissue infarction. Ultimately, if the plaque does not rupture and the lesion continues to grow, the lesion can encroach on the lumen and result in clinically obstructive disease (v). Potential NP therapies in atherosclerosis could benefit from the increased microvessel permeability, which is caused by hypoxia-induced neovascularization of the vasa vasorum and would allow the delivery of NPs to plaques within vascular vessel walls.