Abstract
Dipeptide species are accumulated in the chronic myelogenous leukemia (CML) stem cells [1]. To investigate the molecular mechanisms of the accumulation of dipeptide species in CML stem cells, we performed transcriptome sequencing of long-term stem cells, short-term stem cells, progenitor cells from healthy control and CML-affected mice (GSE70031). The transcriptome data revealed that the expression of a dipeptide transporter (solute carrier family 15, member 2 (SLC15A2)) was elevated only in the CML stem cells. This result indicates that dipeptide species accumulates in CML stem cells through a dipeptide transporter SLC15A2.
Keywords: Chronic myelogenous leukemia, CML stem cell, Dipeptide transporter, RNA sequencing, Slc15a2
| Specifications | |
|---|---|
| Organism/cell line/tissue | Mus musculus/Bone marrow |
| Sex | |
| Sequencer or array type | Illumina HiSeq 2000 |
| Data format | RNA sequencing: raw data (Fastq files) and processed data (tab-delimited text files include RPKM values) |
| Experimental factors | 8 RNA samples for RNA sequencing as follows: 2 samples of normal long-term stem cell 1 sample of normal short-term stem cell 1 sample of KLS− progenitor cell 2 samples of chronic myeloid leukemia long-term stem cell 1 sample of chronic myeloid leukemia short-term stem cell 1 sample of chronic myeloid leukemia KLS− progenitor cell |
| Experimental features | Immature KLS+ cells and KLS− progenitor cells were obtained from healthy control and CML-affected mice by using FACS Aria III cell sorter. |
| Consent | |
| Sample source location | |
1. Direct link to deposited data
2. Experimental design, materials and methods
2.1. RNA sample preparation and transcriptome sequencing
We isolated the most primitive long-term (LT) stem cells (CD150+ CD48− CD135− KLS+ cells), short-term (ST) stem cells (CD150− CD48− CD135− KLS+ cells), and KLS− progenitor cells from healthy littermate control and CML-affected mice. Eight different RNA samples were extracted from two samples of normal LT stem cells, one sample of normal ST stem cells, one sample of normal KLS− progenitor cells, two samples of CML LT stem cells, one sample of CML ST stem cells, and one sample of CML KLS− progenitor cells. Paired-end reads RNA sequencing was performed using Illumina HiSeq2000 for all RNA samples. All sequenced reads were trimmed for adaptor sequence, then mapped to mm9 whole genome using DNAnexus. Reads Per Kilobase of exon per Megabase of library size (RPKM) were calculated using DNAnexus.
2.2. Differentially expressed genes (DEGs)
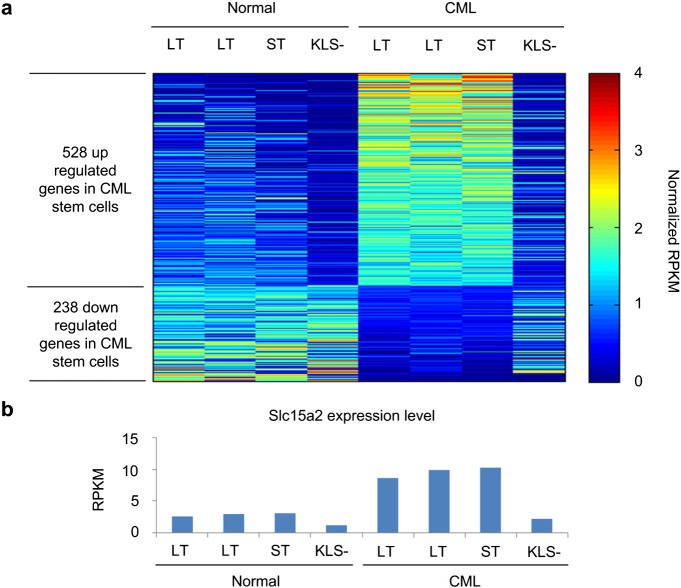
We identified DEGs by comparing expression levels of CML stem cells with those of three other types of cells (normal stem cells, normal KLS− progenitor cells, and CML KLS− progenitor cells). Genes were considered DEGs if their fold-change was more than 2-fold and p-value was less than 0.05. A one-sided two-sample t-test was used to calculate the p-values. From the analysis, we identified 528 up- and 238 down-regulated DEGs in CML stem cells (Fig. 1a). Among up-regulated DEGs, a dipeptide transporter Slc15a2 was highly expressed only in CML stem cells (Fig. 1b). This represents that high expressed Slc15a2 gene causes the accumulation of dipeptide species in CML stem cells.
Fig. 1.
Differentially expressed genes in chronic myelogenous leukemia (CML) cells. (a) Heat map of up- and down-regulated DEGs in CML stem cells. (b) Slc15a2 expression level. LT, ST, and KLS− represent long-term stem cell, short-term stem cell, and KLS− progenitor cell, respectively.
2.3. Gene ontology (GO) analysis
We identified GO terms enriched in the up- and down-regulated DEGs of CML stem cells using DAVID functional annotation tool [1], respectively. GO analysis revealed that the up-regulated DEGs were associated with GO terms “antigen processing and presentation”, “cell adhesion”, “sensory perception of light stimulus”, and “enzyme linked receptor protein signaling pathway” (Table 1). The down-regulated DEGs were associated with GO terms “nucleosome assembly”, “actin cytoskeleton organization”, “immune response”, and “response to nutrient levels” (Table 2).
Table 1.
Gene ontology (GO) terms associated with the up-regulated differentially expressed genes in chronic myelogenous leukemia stem cells.
| Term | p-value | Genes |
|---|---|---|
| GO:0019882 ~ antigen processing and presentation | 1.16E-06 | H2-EA, H2-Q10, MILL2, GM8909, H2-TW3, H2-BL, H2-Q1, EG547347, FCGRT, H2-T10, H2-T24, 1500011B03RIK, H2-DMB2, H2-T3 |
| GO:0007156 ~ homophilic cell adhesion | 5.45E-04 | DSG4, CADM1, FAT2, PCDH9, ROBO2, ESAM, PCDHB12, PCDHGB8, PCDHB21, CDH23, PCDHGA1 |
| GO:0007155 ~ cell adhesion | 5.48E-04 | CADM1, PKHD1, CLDN5, PCDHB12, TGFB2, PCDHGA1, CGREF1, LAMB2, FAT2, ROBO2, ESAM, DPT, CDH23, CNTN5, INPPL1, PCDH9, PCDHGB8, EMILIN2, GPR98, PCDHB21, THY1, NCAM2, DSG4, LAMA3, OTOG, CNTN4, PERP, AOC3 |
| GO:0050953 ~ sensory perception of light stimulus | 0.0033091 | TULP1, PDE6B, EPAS1, ABCA4, DTNBP1, USH2A, GPR98, NYX, CDH23 |
| GO:0007167 ~ enzyme linked receptor protein signaling pathway | 0.0067592 | FGFR2, EGFR, EFNA1, LTBP4, ZFP128, EPHB4, EPHA2, TGFB2, IGSF10, EPHA4, EPHA6, DOK4, PDGFRB, TGFA, PDGFC |
| GO:0007169 ~ transmembrane receptor protein tyrosine kinase signaling pathway | 0.0071313 | IGSF10, EGFR, FGFR2, EPHA4, EPHA6, DOK4, EFNA1, TGFA, PDGFRB, PDGFC, EPHB4, EPHA2 |
| GO:0002474 ~ antigen processing and presentation of peptide antigen via MHC class I | 0.0074334 | H2-Q10, GM8909, H2-TW3, H2-Q1, H2-T3 |
Table 2.
Gene ontology (GO) terms associated with the down-regulated differentially expressed genes in chronic myelogenous leukemia stem cells.
| Term | P-value | Genes |
|---|---|---|
| GO:0006334 ~ nucleosome assembly | 7.60E-08 | HIST1H2AB, HIST1H2BB, HIST1H2BC, HIST1H2BG, A730008H23RIK, HJURP, HIST2H2AC, HIST1H2BJ, HIST3H2A, HIST1H4C, HIST1H3E, HIST1H3F, HIST1H4I, HIST3H2BA |
| GO:0030036 ~ actin cytoskeleton organization | 3.88E-04 | CNN3, MYBPC3, GHRL, SH2B2, EVL, CSRP1, PROX1, DAAM2, CAPN3 |
| GO:0006955 ~ immune response | 0.0045826 | MASP2, IL1RN, MYO1F, RSAD2, TLR5, NLRP3, CXCL10, CFP, H2-T9, OASL1, CD300LG, LBP, CLEC4D |
| GO:0031667 ~ response to nutrient levels | 0.0077562 | UGT1A2, PCSK9, GHRL, VARS, KLF4, LEFTY1 |
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant numbers: HI14C3426 and HI14C2640), and by a grant from the Uehara Memorial Foundation, and a Grant-in-Aid for Scientific Research (B) from MEXT (26290038), Japan.
Reference
- 1.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]